Abstract
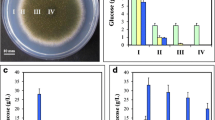
Growth and differentiation of mycelial strands in Rigidoporus lignosus have been shown to depend on suitable combinations of the pH of the media and the nature of the nitrogen and carbon sources. Amino acids as sole nitrogen sources gave rise to vegetative mycelium. At pH 4.5, growth and mycelial strand differentiation required asparagine, as the fungus failed to grow in the absence of this amino acid. However, at pH 6, differentiation of strands occurred appreciably in asparagine-deficient media, suggesting a close balance between pH and amino acid requirements. Ammonium was required for strand differentiation, while nitrate, as a sole nitrogen source, maintained the fungus undifferentiated. Of the carbohydrates tested, only glucose, fructose and mannose supported strand differentiation. Starch was found to be particularly effective in promoting growth of vegetative mycelium. Strand differentiation required more specific conditions than growth of the vegetative mycelium.
Similar content being viewed by others
References
Garraway MO, Evans RC. Fungal Nutrition and Physiology. New York: Wiley, 1984.
Fahmy FA, Yusef HM. A study on certain factors affecting synnema length in Trichurus spiralis Hasselbring. Acta Phytopathol Acad Sci Hung 1974; 9: 89–98.
Weinhold AR, Garraway MO. Nitrogen and carbon nutrition of Armillaria mellea in relation to growth-promoting effects of ethanol. Phytopathology 1966; 56: 108–112.
Wargo PM. Defoliation and secondary-action organism attack: with emphasis on Armillaria mellea. J Arbori 1981; 7: 64–69.
Moody AR, Weinhold AR. Stimulation of rhizomorph production by Armillaria mellea with lipid from tree roots. Phytopathology 1972; 62: 1347–1350.
Shaw CG. In vitro responses of different Armillaria taxa to gallic acid, tannic acid, and ethanol. Plant Pathol 1985; 34: 594–602.
Wargo PM. Changes in phenols affected by Armillaria mellea in bark tissue of roots of oak, Quercus spp. In: Kile GA (ed), Proc. 6th Int. Conf. Root and Butt Rots of Forest Trees; 1983 August 25–31; Melbourne, Victoria, and Gympie, Queensland, Australia. Melbourne: International Union of Forestry Research Organizations. 1984: 198–206.
Lilly VG, Barnett HL. Physiology of the Fungi. New York, McGraw-Hill, 1951.
Jacques-Félix M. Recherches morphologiques, anatomiques, morphogénétiques et physiologiques sur des rhizomorphes de champignons supérieurs et sur le déterminisme de leur formation, II. Recherches sur la morphogenèse des rhizomorphes et télopodes en culture pure. Bull Soc Mycol Fr 1968; 84: 161–307.
Hanlin RJ. Studies on the genus Nectria. I. Factors influencing perithecial formation in culture. Bull Tor Bot Club 1961; 88: 95–103.
Allermann K, Sortkjaer O. Rhizomorph formation in fungi, II. The effect of twelve different alcohols on growth and rhizomorph formation in Armillaria mellea and Clitocybe geotropa. Physiol Plant 1973; 28: 51–55.
Slaughter ]JC. Nitrogen metabolism. In: Berry DR (ed), Physiology of Industrial Fungi. Oxford: Blackwell Scientific Publications, 1988: 58–76.
Garrett SD. Rhizomorph behavior in Armillaria mellea (Vahl) Quél, I. Factors controlling rhizomorph initiation by A mellea in pure culture. Ann Bot 1953; 17: 63–79.
Rykowski K. Recherche sur la nutrition azotée de plusieurs souches de l' Armillaria mellea, I. L'influence de differentes concentrations du carbone et de l'azote (C:N). Eur J Forest Pathol 1976; 6: 264–274.
Botton B, Dexheimer J. Ultrastructure des rhizomorphes du Sphaerostilbe repens B. et Br. Z Pflanzenphysiol 1977; 85: 429–443.
Motta JJ. Peabody DC. Rhizomorph cytology and morphogenesis in Armillaria tabescens; Mycologia 1982; 74: 671–674.
Rayner ADM, Powell KA, Thompson W, Jennings DH. Morphogenesis of vegetative organs. In: Moore D, Casselton LA, Wood DA, Frankland JC (eds), Developmental Biology of Higher Fungi. Cambridge: Cambridge University Press. 1985: 249–279.
Hedger J. Fungi in the tropical forest canopy. Mycologist 1990; 4: 200–202.
Geiger JP, Rio B, Nicole M, Nandris D. Biodegradation of Hevea brasiliensis wood by Rigidoporus lignosus and Phellinus noxius. Eur J For Pathol 1986; 16: 147–159.
Nandris D, Nicole M, Geiger JP. Root rot diseases of rubber trees. Plant Disease 1987; 71: 298–306.
Nicole M, Chamberland H, Rioux D, Lecours N, Rio B, Geiger JP, Ouellette GB. A cytochemical study of extracellular sheaths associated with Rigidoporus lignosus during wood decay. Appl Environ Microbiol 1993; 59: 2578–2588.
Boisson C. Mise en évidence de deux phases mycéliennes successives au cours du développement du Leptoporus lignosus (Kl.) Heim. C R Acad Sc, Paris, Sér. D 1968; 266: 1112–1115.
Boisson C, Goujon M. Induction hormonale et régulation de la formation des sclérotes du Corticium rolfsii (Sacc.) Curzi et des rhizomorphes du Leptoporus lignosus (Kl.) Heim. Rev Cytol Biol Végé 1968; 37: 257–264.
Oso BA. Mycelial growth and amylase production by Talaromyces emersonii. Mycologia 1979; 71: 520–536.
Botton B. Morphogenèse des organes agrégés chez l'ascomycète Sphaerostilbe repens Berk. & Br. [Dissertation]. Nancy, France: University of Nancy I, 1980, 374 pp.
Morquer R, Hubert MF. Métabolisme des glucides et rhizomorphogenèse chez le Clitocybe mellea. C R Acad Sci, Paris, sér. D 1969; 269: 2199–2204.
Weinhold AR. Rhizomorph production by Armillaria mellea induced by ethanol and related compounds. Science 1963; 142: 1065–1066.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Richard, T., Button, B. Growth and mycelial strand production of Rigidoporus lignosus with various nitrogen and carbon sources. Mycopathologia 134, 83–89 (1996). https://doi.org/10.1007/BF00436869
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00436869