Abstract
Results of the present study provide ultrastructural evidence that miracidial morphogenesis is fully completed within the intrauterine eggs while in the most posterior uterine regions of Ityogonimus lorum, a digenean parasite of an Iberian mole, Talpa occidentalis (Eulipotyphla, Talpidae). Using transmission electron microscopy (TEM), the ultrastructural characteristics of diverse cell types and their organelles of these developing embryos and fully formed miracidia within the eggshell were examined. The eggshell and embryonic envelopes are similar to those described previously by many authors for other digeneans. However, the developing miracidia are unique among previously described digeneans in possessing transitory cilia during larvigenesis, but completely lacking cilia in fully formed miracidium larvae. The evidence for completion of miracidial maturation in intrauterine eggs is based on the presence of the following structures: (1) transitional stage of ciliated differentiating miracidial epithelium; (2) apical and lateral glands, characteristic for digenean miracidia; and (3) fully developed germinative cells grouped together in the germinative sac localized in the posterior region of the miracidium. The protonephridial system with its characteristic flame cells and the nervous system with diverse types of neurons and nerve centers, which are characteristic for other digenean species reported until now, are absent from all these developmental stages of I. lorum. Based on these observations, we hypothesize that the life cycle of I. lorum is entirely terrestrial, involving passive transmission by ingestion of eggs containing unciliated miracidia to the first intermediate host.
Similar content being viewed by others
Introduction
Studying digenean eggs actually involves examining three distinct stages of the life cycle: the adult, the embryo, and the miracidium larva, which consequently involves examination of the three distinct processes of fertilization, embryogenesis, and larvigenesis (Conn 2000). Since most studies are conducted in situ within the parent worm, and the uterus, Mehlis’ gland, vitellaria, and other reproductive organs plan a critical role in egg development, the adult must be examined. The eggshell itself is mostly a product of the adult, including the primary shell matrix derived from vitellocyte secretions. Yet, some of the egg coverings are derived from embryonic structures, the inner and outer embryonic envelopes (Conn et al. 2018). Some digeneans are oviparous and do not complete embryogenesis or larvigenesis inside the adult, but rather in the external environment. However, many species are ovoviviparous, completing both embryogenesis and larvigenesis within the parent’s uterus, and thus being released into the environment ready to hatch and swim to seek the host immediately. The best known of these are the schistosomes, which were among the first to be studied due to their medical importance (Świderski 1994a). Because most such digeneans must swim freely through water to locate their snail host, they are equipped with well-developed cilia for locomotion, sensory capabilities integrated by larval neurons, and a protonephridial system with flame cells for osmoregulation in the hypotonic environment (Świderski et al. 2010, 2014; Świderski and Conn 2014). However, some digeneans do not hatch in the water but hatch only inside the first intermediate host snail after ingestion. This is particularly true of those that complete their entire life cycle in a terrestrial environment, using terrestrial snails as intermediate hosts. Most members of the family Brachylaimidae that have been studied with regard to their life cycle are known or suspected to have such host sequences (Ulmer 1951a, b; Mas-Coma et al. 1986; Mas-Coma and Montoliu 1986, 1987, 1995; Butcher and Grove 2001; Gracenea and González-Moreno 2002; González-Moreno and Gracenea 2006; Segade et al. 2011; Sirgel et al. 2012; Nakao et al. 2017, 2018; Waki et al. 2020). Like many members of the family Brachylaimidae, Ityogonimus lorum belongs to this latter category.
Unfortunately, brachylaimids, like most digeneans, have highly resistant eggshells that are technically very difficult to study ultrastructurally due to their small size and especially the difficulty in getting fixatives and embedding resins to infiltrate through the thick tanned eggshell matrix. Our previous success in surmounting these difficulties (see review by Conn et al. 2018) enabled us to obtain suitably infiltrated and embedded eggs. Thus, we engaged in this study to determine whether the miracidia of I. lorum conform to a pattern of functional morphology and ultrastructure particularly suited to an exclusively terrestrial life cycle that would be likely in the case of trematodes that successfully use fossorial moles as definitive hosts.
Materials and methods
Specimens
Live specimens of Ityogonimus lorum (Dujardin, 1845) were isolated from the intestine of a naturally infected Iberian mole (Talpa occidentalis Cabrera, 1907) trapped accidentally in June 2016 during a vole pest control campaign in Priesca (Asturias, Spain).
Transmission electron microscopy
Recovered adult flukes were rinsed with a 0.9% NaCl solution and fixed in cold (4 °C) 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer at pH 7.4 for 2 h, rinsed in 0.1 M sodium cacodylate buffer at pH 7.4, post-fixed in cold (4 °C) 1% osmium tetroxide with 0.9% potassium ferricyanide in the same buffer for 1 h, rinsed in Milli-Q water (Millipore Gradient A10), dehydrated in an ethanol series and propylene oxide, embedded in Spurr’s resin, and polymerized at 60 °C for 72 h. Ultrathin sections (60–90 nm thick) were obtained using a Reichert-Jung Ultracut E ultramicrotome. Sections were placed on 200-mesh copper and gold grids. Sections placed on copper grids were double-stained with uranyl acetate and lead citrate according to the Reynolds procedure (Reynolds 1963). Stained ultrathin sections were examined in a JEOL 1010 transmission electron microscope operated at an accelerating voltage of 80 kV, in the “Centres Científics i Tecnològics” of the University of Barcelona (CCiTUB).
Cytochemistry
Sections placed on gold grids were treated according to the Thiéry test (Thiéry 1967) to reveal the ultrastructural localization of glycogen. Thus, sections were treated in periodic acid (PA), thiocarbohydrazide (TCH), and silver proteinate (SP) as follows: 30 min in 10% PA, rinsed in Milli-Q water, 24 h in TCH, rinsed in acetic solutions and Milli-Q water, 30 min in 1% SP in the dark, and rinsed in Milli-Q water. Ultrathin sections were also examined in a JEOL 1010 transmission electron microscope in the CCiTUB.
Results
Ultrastructure of the advanced stages of embryogenesis and miracidial larvigenesis in the eggshell-enclosed, intrauterine eggs of Ityogonimus lorum was examined by means of transmission electron microscopy (TEM) and TEM cytochemistry.
The four diagrams on Fig. 1a–d illustrate and summarize the four consecutive stages of embryogenesis and larvigenesis observed in the intrauterine eggs of I. lorum. Figure 1a illustrates the ultrastructure of a newly formed egg of I. lorum in the ootype region, which is composed of a fertilized ovum and several vitellocytes all surrounded by a thin, malleable, and discontinuous layer of the differentiating eggshell. The proximal region of the uterus usually contains the eggs in the early stages of their embryonic development (Fig. 1b), composed of several micromeres, which will form the miracidium, as well as macro- and mesomeres participating in the formation of the outer and inner envelopes of the embryo. In this stage, the presence of three surrounding layers was observed: (1) a thick, electron-dense layer of the eggshell; (2) an outer envelope containing two large, slightly flattened nuclei of the macromeres, the cytoplasm of which is forming by fusion into a syncytium; and (3) the three, slightly smaller and less elongated nuclei of the mesomeres, the cytoplasmic fusion of which produces a thick layer of the inner embryonic envelope. In I. lorum, as in numerous other trematodes, the outer envelope with its two flattened nuclei of macromeres disappears rapidly and its place is taken by a rapidly growing inner envelope which accumulates great amounts of nutritive reserves in the form of large lipid droplets and dense agglomerations of beta-glycogen particles (Figs. 2b, 3a, b, 4a, b, 5b, and 6a). The remnants of the outer egg envelope are present and situated just under the eggshell (Fig. 2a, b). Large, concentric profiles of the granular endoplasmic reticulum in the cytoplasmic layer of the inner envelope are visible during an early premiracidial stage of embryogenesis (Fig. 2a). The surface of embryonic tegument contains several vesicles of different sizes, but is still unciliated (Fig. 2a).
Four consecutive stages of embryogenesis and larvigenesis in the intrauterine eggs of Ityogonimus lorum. We added shading and coloration to aid in interpreting important features of each layer. a A newly formed egg of I. lorum in the ootype region, composed of a fertilized ovum (Ov) and several vitellocytes (VC), all surrounded by a thin discontinuous layer of the differentiating eggshell (ES). b The early embryo composed of several micromeres (Mi) in the stage of forming two embryonic envelopes, the outer (OE) and inner (IE) envelopes. c Advanced ciliated stage of miracidial differentiation and maturation. Note the presence of ciliated tegument (CT), a single apical gland (AG), two lateral glands (LG), and numerous undifferentiated miracidial cells (MC). d Final stage of mature non-ciliated larvae. Note the presence of numerous somatic miracidial cells (SC) and several germinative cells (GC), grouped together in a sac-like germinative follicle (GS). In this stage, note also the complete autolysis of the ciliated tegument of the miracidium, entirely eliminated in the presence of several lysosome-like structures, appearing as areas of focal degradation (FCD). MaN, macromere nucleus; MeN, mesomere nucleus; Op, operculum
Ultrastructural details of Ityogonimus lorum egg envelopes. a Two large, concentric profiles of the granular endoplasmic reticulum (GER) in the cytoplasmic layer of the inner egg envelope (IE). The surface of embryonic tegument contains several vesicles of different sizes but is still unciliated. b A thick layer of the inner egg envelope (IE) cytoplasm with large, moderately saturated lipid droplets (L) and large accumulations of beta-glycogen particles (β-gl). The tegumental layer of the differentiating miracidium (Mir) shows presence of numerous ciliary rootlets (CR) and cilia (C), which are separated into individual zones by numerous tegumental processes (TP). ES, eggshell; OE, remnants of the outer envelope. Scale bars = 1 μm
Intrauterine eggs of Ityogonimus lorum illustrating part of the advanced ciliated stage of the miracidium in the advanced stage of differentiation and maturation. a Egg with the larval surface illustrating the transitory development of cilia on the tegumental larval surface, with zones of cilia (C) which are separated by zones of thin, elongated tegumental processes (TP). b Specific cytochemical localization of beta-glycogen particles (β-gl) in the inner egg envelope and in the somatic peripheral musculature of differentiating larvae after application of the test of Thiéry. Bl, blastomere; ES, eggshell; L, lipid droplets; m, mitochondrion; MaN, macromere nucleus; MeN, mesomere nucleus; n, nucleolus; N, nucleus; UW, uterine wall. Scale bars = 2 μm
Ultrastructural details of a progressive degeneration of miracidial cilia at one pole of the Ityogonimus lorum eggs. a Presence of the unciliated, smooth miracidial surface of the operculum-oriented pole of the majority of fully differentiated larvae. b Similar observations at higher magnification, which shows presence of only very limited number of cilia (C) at the pole of egg situated opposite the operculum. AG, apical gland; β-gl, beta-glycogen particles; ES, eggshell; L, lipid droplets; MeN, mesomere nucleus; OE, outer envelope; Op, operculum; UW, uterine wall. Scale bars (a) = 5 μm, (b) = 2 μm
Ultrastructural details of the two types of miracidial glands. a Micrograph showing differences between large, elongated secretory granules SG1 of the apical gland (AG) and spherical, much smaller and evidently less electron-dense granules SG2 of the lateral gland (LG). b Details of the perinuclear region of the apical gland after the test of Thiéry. Note a large nucleus of the apical gland with a prominent, spherical nucleolus (n) surrounded by a thick layer of granular cytoplasm containing several dense accumulations of beta-glycogen particles (β-gl). ES, eggshell. Scale bars (a) = 1 μm, (b) = 2 μm
Ultrastructural details of mature unciliated, smooth-surfaced eggs of Ityogonimus lorum. a Apical gland (AG) with its characteristic, elongated secretory granules. b A germinative cell (GC) with large irregularly shaped nucleus (N) and a prominent, electron-dense nucleolus (n). β-gl, beta-glycogen particles; ES, eggshell; L, lipid droplets; GER, granular endoplasmic reticulum; LG, lateral glands; m, mitochondria; MeN, mesomere nucleus; OE, outer envelope; Op, operculum. Scale bars = 2 μm
In the advanced ciliated stage of the miracidium, a thick layer of the inner envelope cytoplasm with large, moderately saturated lipid droplets and large accumulations of beta-glycogen particles in the cytoplasm of this egg envelope is also observed (Figs. 2b and 3a, b). The tegumental layer of the differentiating miracidium (Fig. 1c) shows the presence of very numerous ciliary rootlets with cilia arising from them, which are separated into individual zones by numerous thin, very long tegumental processes (Figs. 2b and 3a, b). The outer envelope, usually situated between the eggshell and the inner envelope, is no longer visible in this stage (Fig. 3a), but a thick syncytial layer of the inner envelope with large, flattened nuclei of mesomeres, three primary blastomeres which formed initially this egg envelope, usually persists in this layer until advanced stages of embryogenesis (Fig. 3a, b). Numerous lipid droplets and large accumulations of beta-glycogen particles in the cytoplasm of this egg envelope are also observed (Figs. 2b and 3a, b).
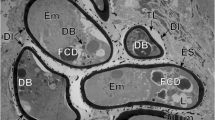
In the advanced stage of differentiation and maturation, a progressive degeneration of miracidial cilia, advancing from one to the opposite pole of the egg, was observed (Fig. 4a, b). This progressive degeneration of cilia on the surface of the miracidial tegument starts much earlier than the autolytic processes of the inner egg envelope, apparently due to a lysosomal activity of the areas of focal cytoplasmic degradation, which appear only much later in the space between the eggshell and the already completely unciliated miracidial tegument (Fig. 7a, b). In the mature eggs, the areas of focal cytoplasmic degradation were frequently observed and may be involved in the autolysis of some embryonic structures. Much longer persistence of the inner envelope is also confirmed and illustrated in Fig. 4a, b and Fig. 6a, b, which show a great variety of cell organelles and inclusions, namely numerous large lipid droplets and dense accumulations of beta-glycogen particles, which represent important reserves of nutritive material for miracidial morphogenesis.
a, b Eggs of Ityogonimus lorum in the final stage of mature intrauterine unciliated miracidia. Note several focal cytoplasmic degradation (FCD) structures between the eggshell (ES) and unciliated miracidial tegument. AG, apical gland; GC, germinative cells; Op, operculum; UW, uterine wall. Scale bars = 2 μm
Two types of miracidial glands are present in the miracidia of I. lorum: the apical gland and two lateral glands (Figs. 1d, 4a, 5a, b, 6a, and 7a). The secretory granules of the apical gland are large and elongated (Fig. 5a), while the secretory granules of the lateral glands are spherical, much smaller, and less electron-dense (Fig. 5a). The apical gland has a large nucleus with a prominent, spherical nucleolus surrounded by a thick layer of granular cytoplasm containing several dense accumulations of beta-glycogen particles (Fig. 5b). The flame cells and nerve cells were never observed during miracidial morphogenesis or in the mature miracidia of I. lorum.
In the mature unciliated, smooth-surface eggs of I. lorum, the germinative cells are grouped together in a sac-like germinative follicle and situated in the medioposterior part of the larva, the germatophore (Fig. 1d). The germinative cells (Figs. 6b and 7b) contain a large irregularly shaped nucleus with prominent, large nucleoli and numerous electron-dense heterochromatin islands arranged in the form of a network or chain-like pattern and distributed mainly in the karyoplasm adjacent to the nuclear membrane. The thin layer of granular cytoplasm is rich in free ribosomes and contains short profiles of granular endoplasmic reticulum and a few small mitochondria. Both nuclear and cytoplasmic features of these cells indicate their great developmental potential for further growth and multiplication in following stages of the life cycle.
Discussion
These results provide the first data ever reported on cellular and subcellular ultrastructure of developing embryos and larvae of a brachylaimid digenean, despite the fact that this family is common worldwide and has been studied extensively by many researchers (e.g., Mas-Coma and Montoliu 1986, 1987, 1995; Butcher and Grove 2001; Segade et al. 2011; Nakao et al. 2017, 2018). As with other digenean taxa, research on eggs, embryos, and miracidium larvae are rare due to the small size, short longevity of these stages, and technical difficulty of preparing the highly resistant intact eggs for electron microscopy, though there is a wealth of information from light microscopy (Conn 2000).
In many basic respects, the structure and formation of Ityogonimus lorum eggs are similar to those of other digeneans (Świderski et al. 2013a; Conn et al. 2018), aspidogastreans (Świderski et al. 2011, 2012), as well as polylecithal cestodes (Świderski 1994b, c; Conn and Świderski 2008; Młocicki et al. 2010), including eggs of the monozoic cestodes such as gyrocotylideans or caryophyllideans (Bruňanská et al. 2012; Levron et al. 2016). Early cleavage leads to separation of blastomeres by size class, with mesomeres and macromeres forming the two embryonic envelopes, and micromeres forming the miracidium larva. The eggshell, as with all trematodes and polylecithal cestodes, is formed in cooperation with the vitellocytes, which are required for eggshell formation to occur (Conn and Etges 1983; Świderski 1994a; Świderski et al. 2010, 2013a). Two pleurogenid digenean species, Brandesia turgida and Prosotocus confusus, have been shown to have an extra “cocoon” layer surrounding each embryo outside the eggshell (Świderski et al. 2013b, 2015), but the functional significance of the “cocoon” is not known and to date has been described only from these two members of one family (Świderski and Conn 2014; Świderski et al. 2014; Conn et al. 2018).
The absence of tegumental cilia in the fully developed miracidium is a feature that is unique among digenean species that have been studied ultrastructurally to date. Our data show that the developing miracidia do have tegumental cilia, and that these are restricted to limited discrete bands so that the full tegument is never ciliated as in most digeneans. Even these small zones of cilia are transitory during larvigenesis, undergoing complete atrophy by the end or miracidial development inside the eggshell, and thus never functioning for locomotion. This has not been described previously for any species of digenean. However, it is similar to many cases of ontogenetic development of structures followed by subsequent atrophy of those structures during early development across the animal kingdom, including vertebrates as described in the classic treatise on evolution by Haeckel (1874).
It is noteworthy that our results show that in I. lorum, the miracidial cilia disappear earlier than the cellular atrophy of the inner egg envelopes, which persist much longer beyond completion of larvigenesis (see Fig. 6a, b). Also, apparently the miracidial cilia disappear progressively, with disappearance starting from one pole of the egg and continuing to the opposite pole on which they are still visible for much longer. Thus, while one pole of the developing miracidium still has cilia until the final stages of larvigenesis, the opposite pole is unciliated from early in larvigenesis (see Fig. 4a, b). For this reason, it is critical that investigators examine the full range of developmental stages when reporting on egg and miracidial structure.
The absence of a protonephridial system in I. lorum is unique among digeneans, and may reflect an exclusively terrestrial life cycle. The capacity for hypertonic osmoregulatory activity in a hypoosmotic freshwater environment is the norm among most digenean miracidia and cercariae, both of which are free-swimming in search of their host in most species. Thus, both of these digenean stages are generally equipped with flame cells and excretory ducts, in both freshwater and marine environments. Although the life cycle of I. lorum is still unknown, our proposal (see below) posits a life cycle in which this species is never outside a host, and thus can rely on the molluscan and mammalian hosts exclusively for their osmoregulatory needs.
Among cestodes, the primarily terrestrial cyclophyllideans typically lack ciliated motile oncosphere stages and also lack protonephridia in the hexacanth (Rybicka 1966; Jabbar et al. 2010), while other primarily aquatic groups such as pseudophyllideans and bothriocephalideans have both of these features (Świderski 1994b), which may be homologous to comparable structures in digeneans (Świderski 1994c). In the context of the present report, it is noteworthy that the strictly aquatic bothriocephalidean cestode, Eubothrium salvelini, has been shown to lack both a ciliated inner embryonic envelope (Świderski et al. 2005) and also protonephridia in the fully formed hexacanth (Młocicki et al. 2010). Thus, the absence of these features, and thus a typical bothriocephalidean coracidium stage, is not always associated with a terrestrial life cycle in the cestodes, but in the case of E. salvelini may be more related to the advanced ovoviviparous development of thin-shelled eggs in that species.
The miracidium of I. lorum is also distinctive among digenean species studied thus far in lacking any element of the nervous system. Similarly, apparent lack of neuronal elements has been described recently for the pleurogenid, B. turgida (Świderski et al. 2014). As with the lack of a protonephridial system, we propose that this is related to the hypothetical life cycle of I. lorum that avoids any active host-seeking behavior in the miracidium. In this regard, it is important to note that the eggs of both B. turgida and I. lorum are ingested by their snail host, and thus do not need neural integration of sensory input. However, while B. turgida infects freshwater snails, and thus may require protonephridia, we hypothesize that I. lorum infects terrestrial snails and thus may not require protonephridia (see Table 1 and Fig. 8).
Based on the unique absence of three crucial host-seeking features in the fully formed miracidia of I. lorum, which lack tegumental cilia, protonephridia, and nervous system, we propose a life cycle as a hypothesis for future studies (Fig. 8). We propose here a cycle that would be completed entirely in a terrestrial environment and would involve two successive terrestrial gastropod hosts. The first would become infected by ingesting intact eggs containing the non-ciliated miracidia from the feces of an infected mole. In this mollusc first-intermediate host, the miracidium would undergo metamorphosis, developing into a sporocyst that would produce numerous cercariae. These would be shed into the mantle cavity of the snail and pass to the outside in the slime trail, from which the cercariae would be ingested by a second-intermediate host snail, in which they would encyst to form metacercariae. The next mole definitive host would become infected by eating the second-intermediate host snail.
Our hypothesis is further strengthened by extensive published research on other brachylaimid species. The first study was done by Ulmer (1951a, b) who reported extensive data on the life cycle of the related brachylaimid of rodents, Postharmostomum helicis, which has a similar two-gastropod life cycle. Ulmer’s P. helicis work in these two reports was reviewed and summarized with additional drawings of the life cycle and individual stages by Olsen (1974). Other authors have demonstrated similar two-gastropod cycles in more recent studies of brachylaimid life cycles, including among others Brachylaima ruminae, a parasite of European rodents (Mas-Coma and Montoliu 1986); Dollfusinus frontalis, a parasite of rodent and soricimorph mammals on Mediterranean islands (Mas-Coma and Montoliu 1987); Pseudoleucochloridium pericardicum, a parasite of soricimorphs in the French Pyrenees (Mas-Coma and Montoliu 1995); Brachylaima aspersae, a parasite of rodents in Spain (Segade et al. 2011); Brachylaima ezohelicis, a parasite of toads in Japan (Nakao et al. 2017); and Brachylaima asakawai, a parasite of rodents in Japan (Nakao et al. 2018).
All of these reports included drawings or light micrographs of eggs containing miracidia, apparently with reduced ciliation. However, all appeared to have some cilia, and thus are in contrast to our current report of I. lorum. It is important to stress that none of these reports employed transmission electron microscopy (TEM), so the identity of small structures such as cilia, which can be confirmed only ultrastructurally, was not possible. Furthermore, those life cycle studies lacking TEM did not examine whether protonephridia or neural cells were present. Thus, the present report is the only one to confirm unciliated miracidia and the first to report lack of protonephridia and neural cells. Future studies of egg ultrastructure in diverse brachylaimid species should address this.
The discovery of several unique features in the embryogeny and larval structure of I. lorum highlights how little we still know about the functional morphology of digeneans, as well as their developmental plasticity in response to diverse environments and strategies to access hosts. This provides a compelling argument for why more research is needed, especially across disparate taxa, and among the earliest stages of ontogeny. Goater et al. (2005) and Conn et al. (2008) presented a case for similar increase of study for the cercaria and post-cercaria stages of digeneans. Based on their interpretation of studies on the strigeid, Ornithodiplostomum ptychocheilius, they proposed recognition of three distinct developmental stages between the cercaria and adult of that species, and argued for more research and open reconsideration of what have been considered typical digenean life cycles (Conn 2007, 2010). Likewise, it is possible that the traditional thinking of miracidium function and role in the life cycle of digeneans has been overly simplified due to insufficient research on diverse taxa inhabiting diverse hosts in diverse environments.
References
Bruňanská M, Mackiewicz JS, Młocicki D, Świderski Z, Nebesářová J (2012) Early intrauterine embryonic development in Khawia sinensis Hsü, 1935 (Cestoda, Caryophyllidea, Lytocestidae), an invasive tapeworm of carp (Cyprinus carpio): an ultrastructural study. Parasitol Res 110:1009–1017. https://doi.org/10.1007/s00436-011-2590-2
Butcher AR, Grove DI (2001) Description of the life-cycle stages of Brachylaima cribbi n. sp. (Digenea: Brachylaimidae) derived from eggs recovered from human faeces in Australia. Syst Parasitol 49:211–221. https://doi.org/10.1023/A:1010616920412
Conn DB (2000) Atlas of invertebrate reproduction and development, 2nd edn. Wiley, New York
Conn DB (2007) Life cycles and biogeography of fish parasites: recent advances and future directions. Parassitologia 49(Suppl. 2):282
Conn DB (2010) Invasion strategies of trematodes involving activity of the life-cycle stages between cercaria and metacercaria. In: Hodová I, Koubková B (eds) 18th helminthological days 2010: book of abstracts. MUNI Press, Masaryk University, Brno, pp 22–23 ISBN: 978-80-210-5244-4
Conn DB, Etges FJ (1983) Inhibition of egg production in an anomalous Plagitura salamandra Holl, 1928 (Trematoda: Plagiorchiidae). J Parasitol 69:784–786
Conn DB, Świderski Z (2008) A standardized terminology of the embryonic envelopes and associated developmental stages of tapeworms (Platyhelminthes: Cestoda). Folia Parasitol 55:42–52. https://doi.org/10.14411/fp.2008.006
Conn DB, Goater CP, Bray D (2008) Developmental and functional ultrastructure of Ornithodiplostomum ptychocheilus diplostomula (Trematoda: Strigeoidea) during invasion of the brain of the fish intermediate host, Pimephales promelas. J Parasitol 94:635–642. https://doi.org/10.1645/GE-1421.1
Conn DB, Świderski Z, Miquel J (2018) Ultrastructure of digenean trematode eggs (Platyhelminthes: Neoophora): a review emphasizing new comparative data on four European Microphalloidea. Acta Parasitol 63:1–14. https://doi.org/10.1515/ap-2018-0001
Goater CP, Bray D, Conn DB (2005) Cellular aspects of early development of Ornithodiplostomum ptychocheilus metacercariae in the brain of fathead minnows, Pimephales promelas. J Parasitol 91:814–821. https://doi.org/10.1645/GE-3485.1
González-Moreno O, Gracenea M (2006) Life cycle and description of a new species of brachylaimid (Trematoda: Digenea) in Spain. J Parasitol 92:1305–1312. https://doi.org/10.1645/GE-821R.1
Gracenea M, González-Moreno O (2002) Life cycle of Brachylaima mascomai n. sp. (Trematoda: Brachylaimidae), a parasite of rats in the Llobregat delta (Spain). J Parasitol 88:124–133. https://doi.org/10.1645/0022-3395(2002)088[0124:LCOBMN]2.0.CO;2
Haeckel E (1874) Anthropogenie oder Entwickelungsgeschichte des Menschen. Engelmann, Leipzig
Jabbar A, Crawford S, Młocicki D, Świderski Z, Conn DB, Jones MK, Beveridge I, Lightowlers MW (2010) Ultrastructural reconstruction of Taenia ovis oncospheres from serial sections. Int J Parasitol 40:1419–1431. https://doi.org/10.1016/j.ijpara.2010.04.011
Levron C, Scholz T, Vancová M, Kuchta R, Conn DB (2016) Ultrastructure of embryonated eggs of the cestode Gyrocotyle urna (Gyrocotylidea) using cryo-methods. Zoomorphology 135:279–289. https://doi.org/10.1007/s00435-016-0310-2
Mas-Coma S, Montoliu I (1986) The life cycle of Brachylaima ruminae n. sp. (Trematoda: Brachylaimidae), a parasite of rodents. Z Parasitenkd 72:739–753. https://doi.org/10.1007/BF00925095
Mas-Coma S, Montoliu I (1987) The life cycle of Dollfusinus frontalis, a brachylaimid trematode of small mammals (lnsectivora and Rodentia). Int J Parasitol 17:1063–1079. https://doi.org/10.1016/0020-7519(87)90159-7
Mas-Coma S, Montoliu I (1995) Life cycle of Pseudoleucochloridium pericardicum n. sp. (Trematoda: Brachylaimidae), a parasite of shrews (lnsectivora: Soricidae) in the Oriental Pyrenees. Res Rev Parasitol 55:155–171
Mas-Coma S, Esteban JG, Valero MA (1986) The genus Scaphiostomum Braun, 1901 (Trematoda: Brachylaimidae): a systematic review and description of Scaphiostomum palaearcticum n. sp. Syst Parasitol 8:141–150. https://doi.org/10.1007/BF00009870
Młocicki D, Świderski Z, Bruňanská M, Conn DB (2010) Functional ultrastructure of the hexacanth larvae in the bothriocephalidean cestode Eubothrium salvelini (Schrank, 1790) and its phylogenetic implications. Parasitol Int 59:539–548. https://doi.org/10.1016/j.parint.2010.07.001
Nakao M, Waki T, Sasaki M, Anders JL, Koga D, Asakawa M (2017) Brachylaima ezohelicis sp. nov. (Trematoda: Brachylaimidae) found from the land snail Ezohelix gainesi, with a note of an unidentified Brachylaima species in Hokkaido, Japan. Parasitol Int 66:240–249. https://doi.org/10.1016/j.parint.2017.01.015
Nakao M, Sasaki M, Waki T, Anders JL, Katahia H (2018) Brachylaima asakawai sp. nov. (Trematoda: Brachylaimidae), a rodent intestinal fluke in Hokkaido, Japan, with a finding of the first and second intermediate hosts. Parasitol Int 67:565–574. https://doi.org/10.1016/j.parint.2018.04.010
Olsen OW (1974) Animal parasites: their life cycles and ecology, 3rd edn. University Park Press, Baltimore
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212. https://doi.org/10.1083/jcb.17.1.208
Rybicka K (1966) Embryogenesis in cestodes. Adv Parasitol 4:107–186. https://doi.org/10.1016/S0065-308X(08)60449-2
Segade P, Crespo C, García N, García-Estévez JM, Arias C, Iglesias R (2011) Brachylaima aspersae n. sp. (Digenea: Brachylaimidae) infecting farmed snails in NW Spain: morphology, life cycle, pathology, and implications for heliciculture. Vet Parasitol 10:273–286. https://doi.org/10.1016/j.vetpar.2010.10.026
Sirgel WF, Artigas P, Bargues MD, Mas-Coma S (2012) Life cycle of Renylaima capensis, a brachylaimid trematode of shrews and slugs in South Africa: two-host and three-host transmission modalities suggested by epizootiology and DNA sequencing. Parasites Vectors 5:169. https://doi.org/10.1186/1756-3305-5-169
Świderski Z (1994a) Origin, differentiation and ultrastructure of egg envelopes surrounding the miracidia of Schistosoma mansoni. Acta Parasitol 39:64–72
Świderski Z (1994b) Origin, differentiation and ultrastructure of egg envelopes surrounding the coracidia of Bothriocephalus clavibothrium (Cestoda, Pseudophyllidea). Acta Parasitol 39:73–81
Świderski Z (1994c) Homology and analogy in of egg envelopes surrounding the coracidia of Bothriocephalus clavibothrium and miracidia of Schistosoma mansoni. Acta Parasitol 39:123–130
Świderski Z, Conn DB (2014) Comparative ultrastructure of the intrauterine eggs of four European trematodes. In: Oros M, Vasilková Z (eds) V4 parasitological meeting: parasites in the heart of Europe. Slovak Society for Parasitology at SAS, Kosice, pp 27–28 ISBN: 978-80-968473-7-2
Świderski Z, Bruňanská M, Młocicki D, Conn DB (2005) Ultrastructure of the oncospheral envelopes in the pseudophyllidean cestode Eubothrium salvelini (Schrank, 1790). Acta Parasitol 50:312–318
Świderski Z, Bakhoum AJS, Młocicki D, Miquel J (2010) Ultrastructural studies on egg envelopes surrounding the miracidia of Mediogonimus jourdanei Mas-Coma et Rocamora 1978 (Digenea, Microphalloidea, Prosthogonimidae). Acta Parasitol 55:233–245. https://doi.org/10.2478/s11686-010-0031-5
Świderski Z, Poddubnaya LG, Gibson DI, Levron C, Młocicki D (2011) Egg formation and the early embryonic development of Aspidogaster limacoides Diesing, 1835 (Aspidogastrea: Aspidogastridae), with comments on their phylogenetic significance. Parasitol Int 60:371–380. https://doi.org/10.1016/j.parint.2011.06.006
Świderski Z, Poddubnaya LG, Gibson DI, Młocicki D (2012) Advanced stages of embryonic development and cotylocidial morphogenesis in the intrauterine eggs of Aspidogaster limacoides Diesing, 1835 (Aspidogastrea), with comments on their phylogenetic implications. Acta Parasitol 57:131–148. https://doi.org/10.2478/s11686-012-0025-6
Świderski Z, Miquel J, Montoliu I, Feliu C, Gibson DI (2013a) Ultrastructure of the intrauterine eggs of the microphallid trematode Maritrema feliui: evidence of early embryonic development. Parasitol Res 112:3325–3333. https://doi.org/10.1007/s00436-013-3512-2
Świderski Z, Poddubnaya LG, Zhokhov AE, Miquel J, Gibson DI (2013b) An ultrastructural study of the egg wall surrounding the miracidium of the digenean Brandesia turgida (Brandes, 1888) (Plagiorchiida: Pleurogenidae), with the description of a unique cocoon-like envelope. Zool Anz 253:114–118. https://doi.org/10.1016/j.jcz.2013.09.001
Świderski Z, Poddubnaya LG, Zhokhov AE, Miquel J, Conn DB (2014) Ultrastructural evidence for completion of the entire miracidial maturation in intrauterine eggs of the digenean Brandesia turgida (Brandes, 1988) (Plagiorchiida: Pleurogenidae). Parasitol Res 113:1103–1111. https://doi.org/10.1007/s00436-013-3747-y
Świderski Z, Miquel J, Torres J, Conn DB (2015) Ultrastructural study of the egg wall surrounding the developing miracidia of the digenean Prosotocus confusus (Looss, 1894) (Plagiorchiida: Pleurogenidae), with the description of a unique cocoon-like envelope. Parasitol Res 114:185–191. https://doi.org/10.1007/s00436-014-4177-1
Thiéry JP (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microsc (Paris) 6:987–1018
Ulmer MJ (1951a) Postharmostomum helicis (Leidy, 1847) Robinson 1949 (Trematoda), its life history and a revision of the subfamily Brachylaeminae. Part I. Trans Amer Microsc Soc 70:189–238
Ulmer MJ (1951b) Postharmostomum helicis (Leidy, 1847) Robinson 1949 (Trematoda), its life history and a revision of the subfamily Brachylaeminae. Part II. Trans Amer Microsc Soc 70:319–347
Waki T, Sasaki M, Mashino K, Iwaki T, Nakao M (2020) Brachylaima lignieuhadrae n. sp. (Trematoda: Brachylaimidae) from land snails of the genus Euhadra in Japan. Parasitol Int 74:101992. https://doi.org/10.1016/j.parint.2019.101992
Acknowledgments
The authors are grateful to Marcos Miñarro (SERIDA) and Roser Adalid for their valuable help in the fieldwork. The authors also wish to thank the personnel of the “Unitat de Microscòpia Electrònica, Facultat de Medicina, Centres Científics i Tecnològics de la Universitat de Barcelona (CCiTUB)” for their support in the preparation of samples. JM is a member of the 2017-SGR-1008 research group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Świderski, Z., Conn, D.B. & Miquel, J. Ultrastructure and cytochemistry of intrauterine embryonic and larval stages of Ityogonimus lorum (Digenea: Brachylaimidae) involving transitory development of ciliated miracidia. Parasitol Res 119, 1583–1595 (2020). https://doi.org/10.1007/s00436-020-06629-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06629-z