Abstract
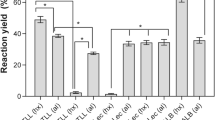
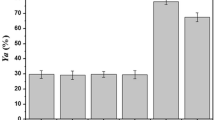
Lipases are enzymes used in numerous reactions of industrial interest. Depending on their aqueous microenvironment, lipases can catalyze hydrolysis or, conversely, organic synthesis like interesterification. This reaction can be used as a method to modify the physical and chemical properties of fats and oils, a basic process for production of “structured lipids”. For such synthesis reactions, thermodynamic water activity (aw) of the catalyst is generally the most important parameter to control. Actually, it will directly determine the performance of the synthesis, namely its yield, selectivity and stability. Effect of the aw on the activity of immobilized Thermomyces lanuginosus and Candida antarctica B lipases in interesterification reactions was studied. Water sorption and desorption isotherms were determined, showing a phenomenon of hysteresis for the Thermomyces lanuginosus lipase. Evaluation of the influence of aw on reaction yields revealed that the IE activity tends to increase with the water activity of immobilized Thermomyces lanuginosus lipase. In contrast, aw had little influence in the case of the Candida antarctica B lipase.


Similar content being viewed by others
References
Hasan F, Shah AA, Hameed A (2006) Industrial applications of microbial lipases. Enzyme Microb Technol 39(2):235–251
Houde A, Kademi A, Leblanc D (2004) Lipases and their industrial applications—an overview. Appl Biochem Biotechnol 118(1–3):155–170
Fickers P, Destain J, Thonart P (2008) Lipases are atypical hydrolases: principal characteristics and applications. Biotechnologie Agronomie Société Et Environnement 12(2):119–130
Xu X (2000) Production of specific-structured triacylglycerols by lipase-catalyzed reactions: a review. Eur J Lipid Sci Technol 102(4):287–303
Villeneuve P (2007) Lipases in lipophilization reactions. Biotechnol Adv 25(6):515–536
Petersson AEV, Adlercreutz P, Mattiasson B (2007) A water activity control system for enzymatic reactions in organic media. Biotechnol Bioeng 97(2):235–241
Xu X (2005) Modification of oils and fats by lipase-catalyzed interesterification: aspects of process engineering, in enzymes in lipid modification. Wiley-VCH Verlag GmbH & Co. KGaA. p 190–215
Schmid U, Bornscheuer UT, Soumanou MM, McNeill GP, Schmid RD (1998) Optimization of the reaction conditions in the lipase-catalyzed synthesis of structured triglycerides. J Am Oil Chem Soc 75(11):1527–1531
Caro Y, Pina M, Turon F, Guilbert S, Mougeot E, Fetsch DV, Attwool P, Graille J (2002) Plant lipases: biocatalyst aqueous environment in relation to optimal catalytic activity in lipase-catalyzed synthesis reactions. Biotechnol Bioeng 77(6):693–703
Xia X, Wang C, Yang B, Wang Y-H, Wang X (2009) Water activity dependence of lipases in non-aqueous biocatalysis. Appl Biochem Biotechnol 159(3):759–767
Svensson I, Wehtje E, Adlercreutz P, Mattiasson B (1994) Effects of water activity on reaction rates and equilibrium positions in enzymatic esterifications. Biotechnol Bioeng 44(5):549–556
Chowdary GV, Prapulla SG (2002) The influence of water activity on the lipase catalyzed synthesis of butyl butyrate by transesterification. Process Biochem 38(3):393–397
Osorio NM, Dubreucq E, Da Fonseca MMR, Ferreira-Dias S (2009) Lipase/acyltransferase-catalysed interesterification of fat blends containing n-3 polyunsaturated fatty acids. Eur J Lipid Sci Technol 111(2):120–134
Cambon E, Gouzou F, Pina M, Barea B, Barouh N, Lago R, Ruales J, Tsai SW, Villeneuve P (2006) Comparison of the lipase activity in hydrolysis and acyl transfer reactions of two latex plant extracts from babaco (Vasconcellea × Heilbornii Cv.) and Plumeria rubra: effect of the aqueous microenvironment. J Agric Food Chem 54(7):2726–2731
Greenspan L (1977) Humidity fixed points of binary saturated aqueous solutions. J Res Nat Bur Stand 81A(1):89
Halling PJ (1994) Thermodynamic predictions for biocatalysis in non conventional media: theory, tests and recommendations for experimental design and analysis. Enz Microb Technol 16:178–206
Valivety RH, Halling PJ, Macrae AR (1992) Reaction rate with suspended lipase catalysts shows similar dependence on water activity in different organic solvents. Biochim Biophys Acta 1198:218–222
Svensson I, Wehtje E, Adlercreutz P, Mattiasson B (1994) Effects of water activity on reaction rates and equilibrium positions in enzymatic esterifications. Biotechnol Bioeng 44:549–556
Dudal Y, Lortie R (1995) Influence of water activity on the synthesis of triolein catalyzed by immobilized Mucor miehei lipase. Biotechnol Bioeng 45:129–134
Acknowledgments
This study was performed in the framework of a Ph.D. study with financial support from the St Hubert Company in France.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pérignon, M., Lecomte, J., Pina, M. et al. Activity of immobilized Thermomyces lanuginosus and Candida antarctica B Lipases in Interesterification Reactions: Effect of the Aqueous Microenvironment. J Am Oil Chem Soc 90, 1151–1156 (2013). https://doi.org/10.1007/s11746-013-2256-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-013-2256-6