Abstract
This work was carried out to evaluate the antagonism of Trichoderma isolates to the soil-borne plant pathogenic fungi Sclerotinia sclerotiorum and Sclerotium rolfsii. Thirty-four antagonist fungal strains were used. In the dual culture tests, Trichoderma isolates CEN281 and CEN287 stood out, both belong to the species T. afroharzianum and originate from soil cultivated with cotton. The folding, deformation and hyphal penetration effects of the pathogens were confirmed under microscopy in all the Trichoderma isolates. In the volatile metabolites production assays, no inhibition of S. sclerotiorum growth was observed. On the other hand, six isolates were able to inhibit S. rolfsii, including CEN1075 (T. asperellum) which originates from the tomato rhizosphere. As for the production of non-volatile metabolites, 11 isolates, mostly obtained from soil samples from the tomato rhizosphere, showed action against both pathogens. Regarding the damping-off of tomato seedlings caused by S. rolfsii, it was observed that the Trichoderma isolates CEN281 and CEN126 were the ones that most suppressed the disease, and both were isolated from the rhizosphere of this plant. This work allowed the selection of new Trichoderma isolates with potential antagonistic to the important soil fungi in question. The isolates CEN281, CEN1070 and CEN1080 showed the best results in controlling the damping-off of tomato seedlings and, therefore, will be evaluated in field studies.
Similar content being viewed by others
Introduction
The tomato plant (Solanum lycopersicum L.), belonging to the Solanaceae family, is probably native to the Andean Region, western part of South America; was domesticated in Mexico and distributed to other regions of the globe (Knapp and Peralta 2016), until reaching Brazil. Among the vegetables, the tomato stands out, due to the volume of production and jobs generated in its production (Machado et al. 2018). However, to meet the market demand for new products, intensive research work is required to select efficient antagonists against soil-borne pathogens, including Sclerotinia sclerotiorum (Lib.) de Bary - white mold, and Sclerotium rolfsii Sacc. - sclerotium rot (Singh et al. 2017).
Plant diseases are among the main causes of agricultural losses worldwide and their management is a challenge in the food security of the world population (Nelson 2020). Several approaches have been recommended to prevent or mitigate the effects caused by plant pathogens, including agronomic and sanitation practices (Thambugala et al. 2020; Asad 2022). The experience of controlling plant diseases, with the exclusive use of chemical fungicides, has already proved to be unfeasible, especially in the case of soil-borne pathogens (Smolińska and Kowalska 2018), in addition, such products can also have negative effects on the health of consumers and rural workers, damage the environment and, over time, result in resistance to these products (Zubrod et al. 2019). This fact further aggravates the health problems for which such products are indicated. To combat this menace, use of biological control agent for the management of plant diseases is the best possible alternative (Akhtar and Javaid 2018).
Trichoderma is a genus of anamorphic fungi, with the sexual (teleomorphic) phase in Hypocrea (Hypocreales, Ascomycota), originally introduced by Persoon (1794). These fungi have outstanding importance as bioregulators and natural antagonists of soil-borne phytopathogenic fungi, due to their great competitive capacity and a diversity of mechanisms of action (Benítez et al. 2004; Jaiswa and Khadk 2020). The growing concern about the effects of synthetic fungicides on the environment and the residues of such products in food has reinforced the interest in the use of fungi of the Trichoderma genus in agriculture (Abdelkhalek et al. 2022) and study of its occurrence (Silva et al. 2020a, b). In Brazil, according to the platform of the Ministry of Agriculture, Livestock and Supply, there are currently about 58 Trichoderma-based products registered as biofungicides (Agrofit 2023). It is important to note that some of the isolates used as active ingredients in these products are repeated under different trade names. The variations of products with the same isolates are caused by changes in the formulation, target and concentration of spores, therefore, it is important to search for isolates from different species for use in the composition of new bioproducts.
In the routine for selection of Trichoderma isolates for biological control, it is necessary to carry out in vitro tests in paired culture, in addition to verifying the production of volatile and non-volatile metabolites with antimicrobial activity and verification of hyperparasitic interactions by microscopic examination (Asad et al. 2022). Data related to the dual cultures of S. rolfsii and S. sclerotiorum with Trichoderma spp. have been published by several authors, such Marques et al. 2016, 2022b; Mesquita et al. (2017); Sumida et al. (2018), and by Kamel et al. (2020). Regarding studies on the production of volatile and non-volatile metabolites that are active against these two pathogens, the works by Louzada et al. (2016); Mesquita et al. (2017); Kushwaha et al. (2018); Marques et al. 2018, 2022a; Silva et al. (2020a, b, 2021) stand out. Regarding the verification of the hyperparasitism of Trichoderma isolates to phytopathogenic fungi, the works of Louzada et al. (2009), Troian et al. (2014), and by Kamel et al. (2020) are of interest. In vitro studies are useful to verify the mechanism of action of antagonists and to distinguish isolates for in vivo studies, aimed at the selection of potential biocontrol agents (BCAs) and possible use in commercial formulations.
Studies have already linked the use of fungi of the genus Trichoderma in the in vivo control of diseases caused by pathogens in tomato fields (Abdelkhalek et al. 2022). Regarding studies with Trichoderma in the control of sclerotium rot in tomato, it can be mentioned those carried out by Islam et al. (2016), Suriyagamon et al. (2018), Kamel et al. (2020) and Blanco et al. (2021). However, there are no recommended products for this pathogen in tomato crops in Brazil (Agrofit 2023). Keeping in view of the importance of routine studies of prospection and characterization of Trichoderma isolates, mainly for soil-borne and difficult-to-control pathogens, the main objective of this work was to isolate, select and evaluate fungi of the genus Trichoderma in vitro against the pathogens that cause white mold and sclerotium rot in vivo.
Materials and methods
Isolates from pathogens and antagonists
The work was carried out at the Phytopathology Laboratory of the Biological Control Building of Embrapa Recursos Genéticos e Biotecnologia (CENARGEN), Brasília-DF (Distrito Federal), Brazil. Thirty-four Trichoderma isolates were used, of which 13 were obtained from soil samples collected in a tomato cultivation area at Embrapa Hortaliças (CNPH), located in Gama (DF), and the other isolates were already deposited in the Collection of Fungi for Biological Control of Embrapa Recursos Genéticos e Biotecnologia. The other 21 isolates were selected based on preliminary results obtained in the control of white mold (S. sclerotiorum) and bean collar rot (S. rolfsii) by other team members. Among the isolates used, ten were identified at the species level, as shown in Table 1, and some were characterized in a previous work regarding growth promotion in tomato plants (Montalvão et al. 2020).
In the biocontrol assays, isolates CEN217 and CEN216 of S. sclerotiorum and S. rolfsii were used, respectively. These are also stored in the Embrapa collection.
Dual culture assessment
Antagonistic potential was assessed by the paired culture technique (Dennis and Webster 1971a). Isolates from both pathogens and antagonists were multiplied in PDA (Potato-Dextrose-Agar) medium at 25ºC and 12 h of photoperiod, for seven days. For this experiment, agar discs colonized by fungi were used, which were placed on opposite sides of the plates. As a control treatment, PDA plates containing only the pathogen were prepared. For each Trichoderma isolate, three replications were performed. The evaluations were carried out at seven days of cultivation, assigning scores according to the scale of note discribed by Bell et al. (1982).
The structures of the confrontation zones of the two fungi were analyzed with the aid of an Optical Microscope (OM) (Olympus BX40), with a 40X magnifying glass. For that, slides containing fragments of these structures of confrontation between the fungi were made and observed for the presence of curling, hyphal plasmolysis, growth of parallel hyphae, as well as structural alterations of these hyphae.
A completely randomized design was used, with three replications. They were performed twice.
Antagonistic interactions under the scanning electron microscope
Detailed studies of the interaction between Trichoderma isolates and pathogens were carried out by Scanning Electron Microscopy (SEM), using samples from paired culture, with 11 isolates of the antagonist. For this purpose, discs of PDA (5 mm in diameter) from areas of confrontation between colonies of isolates of Trichoderma spp. and pathogens (S. sclerotiorum and S. rolfsii) were removed and submitted to the procedure described by Bossola and Russell (1998), adapted by Alves (2004).
Volatile metabolites production
To evaluate the inhibitory effect of volatile metabolites, tests were performed according to the methodology described by Silva et al. (2020a, b). Where two plates were overlapping, the bottom one with Trichoderma and the top one with the phytopathogen, after 7 h of previous cultivation. To prevent gases from leaking out, the overlapping plates were tightly sealed with PVC film. Incubation was carried out in the same way as described above. The control treatment were plates with PDA containing the pathogen and another without the antagonist. Mycelial growth assessments were performed when the entire surface of the medium in the control plates, that is, in those whose lower bases contained PDA medium, were covered by the pathogen.
The tests were carried out in a completely randomized design, with three replications and twice.
Non-volatile metabolites production
Tests to evaluate the inhibition of the growth of the pathogen by non-volatile metabolites were performed based on the methodology described by Carvalho et al. (2021), with modifications. For this test, the antagonist isolates were grown in Erlenmeyer flasks (250 mL) containing 100 mL of potato-dextrose-based liquid medium. As inoculum, 5 agar discs (5 mm in diameter) were used per plate, taken from cultures of Trichoderma grown in PDA, with five days of age. Incubation was performed in an orbital shaker, at 150 rpm, at a temperature of 25 ± 2ºC, in the absence of light. After seven days, the liquid part was collected by filtration through filter paper and was filtered again with the aid of cellulose membranes of 0.45 μm. The filtrate thus sterilized was incorporated into the autoclaved PDA medium at a rate of 25% (v/v). For each Trichoderma filtrate, three plates were prepared. Petri dishes with the medium received a culture disc of the pathogen at the center and were then kept at 25 ± 2 °C. As a control, plates with PDA medium without the filtered cultures of the antagonist were used. In this experiment, radial mycelial growth measurements were also performed only when the control plate had been completely taken up by the phytopathogen.
The tests were carried out in a completely randomized design, with three replications and twice.
Suppression of tomato seedlings damping-off in greenhouse
The experiment was carried out in a greenhouse maintained at a temperature of 14–35 ºC and humidity around 80%, with a photoperiod of 12 h. Trichoderma isolates selected in the previous assays were cultivated in PDA medium at a temperature of 25 ± 2°Cin the presence of light for seven days (Table 2). Culture spores were extracted by adding 20 ml of aqueous Tween 80 solution (0.05) to Petri dishes. The spore suspensions obtained were adjusted to a concentration of 6 × 107 conidia mL− 1. The multiplication of the pathogens was carried out according to a methodology adapted from Serra and Silva (2005).
‘San Vito’ tomato seeds were microbiolized with Trichoderma isolates and allowed to germinate in gerbox boxes placed in an incubator with a 12-hour photophase and a temperature of 25 ± 2 °C. Controls were also pre-germinated under the same conditions as above, but without microbiolization with Trichoderma isolates.
After producing antagonist, pathogen and host, the assay was set up as follows: 400 mL of autoclaved soil packed in disposable cups received 20 mL of conidia suspension (6 × 107 conidia mL− 1) and, 48 h later, 4 g of rice colonized by the pathogen. Three days later, five germinated tomato seeds were distributed per cup. Two control treatments were included, one with the pathogen and without Trichoderma (control 1) and the other without the pathogen and without Trichoderma (control 2). After 11 and 17 days of cultivation, the growth of tomato plants, the mortality rate and the presence of lesions were evaluated. For each Trichoderma isolate, three replicates were used.
The experimental design was completely randomized, with three replications (pots with five tomato seedlings). The experiments were performed twice.
Silhouette validation method
The Silhouette Validation Technique, according to Rousseeuw (1987), started from the premise by which the silhouette length is calculated for each sample, its average width for each cluster, its global average and the average for the total data set. Thus, with this approximation, each cluster can be represented by the so-called silhouette, based on the comparison of its narrowing and separation. The mean silhouette length was applied to validate the cluster and decide the ideal number of clusters.
The silhouette S(i) construction is given as:
where a(i) is the average dissimilarity of object i in relation to all other objects in the same cluster; (bi) is the minimum average dissimilarity of object i in relation to all other clusters (in the closest cluster).
It is followed by the formula − 1 ≤ S(i) ≤ 1. If the silhouette value is close to 1, it indicates that the sample is well clustered and it has been assigned an opportune cluster. If its value is close to zero, it means that the sample could be allocated to another cluster closer, and it is equally distant from both clusters. If the silhouette value is -1, it indicates that the sample was misclassified. The average silhouette length for all objects is simply the average of S(i).
Statistical analyzes were performed using the “R” Statistical Program.
Results
Dual culture assessment
Regarding the in vitro tests against S. sclerotiorum, seven groups of isolates were formed (Table 3), where the mean silhouette length was 0.94, indicating a good separation. In this case, the best inhibition result was observed for group 7, where isolate CEN219 is allocated, followed by the isolates of group 6, which comprises CEN141, CEN223, CEN281 and CEN287. Group 1 was the third best inhibitor and accommodates isolates CEN1068, CEN155, CEN161, CEN170 and CEN273. Group 2 consisted of isolates CEN1069, CEN1071, CEN1074, CEN126, CEN194, CEN280 and CEN289 and was followed by group 3 (CEN1070, CEN1073, CEN1075, CEN129, CEN162 and CEN201); next, appear group 5 (CEN1077, CEN1078, CEN169, CEN209, CEN290 and CEN316) and group 4 (CEN1072, CEN1076, CEN1079, CEN1080 and CEN288), which presented the least satisfactory results, although differing from the control that formed group 8.
Concerning the inhibition of S. rolfsii in paired culture, seven groups of isolates were also formed, as shown in Table 3. The mean silhouette length was 0.91, which indicates a good separation of the groups. According to the silhouette method, the tested isolates were divided into groups according to their efficiency in inhibiting the mycelial growth of the pathogen, where the best result was achieved by group 2 composed of isolates CEN1069, CEN1071, CEN129, CEN169, CEN170, CEN194, CEN273, CEN281, CEN287 and CEN289; the second best result was obtained by group 1, which comprises isolates CEN1068, CEN1077, CEN126, CEN141 and CEN316; in third place was group 3, where the isolates are CEN1070, CEN1073, CEN1074, CEN1076, CEN1078, CEN155, CEN161 and CEN162, followed by group 5 (CEN1075, CEN1079, CEN1080, CEN201, CEN209, CEN219 and CEN280), group 6 (CEN288), group 4 (CEN1072 and CEN223), group 7 (CEN290) and finally group 8, formed only by the control treatment.
Interaction of pathogens and Trichoderma spp. under optical and scanning electron microscopy
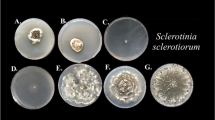
In the dual culture carried out between the 34 isolates of Trichoderma and isolates CEN217 (S. sclerotiorum) and CEN216 (S. rolfsii), antagonistic interactions were observed, typically due to competition and hyperparasitism. As a result of competition, there was entanglement and deformation of hyphae (Figs. 1 and 2), as observed in light microscopy. It was also observed in some cases that Trichoderma isolates penetrated and colonized propagules (sclerotia) of S. sclerotiorum and S. rolfsii isolates.
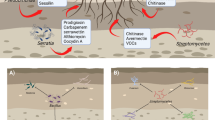
Due to the impossibility of carrying out observations using a scanning electron microscope (SEM) with all the tests performed, 11 of them were chosen, involving both the pathogens S. sclerotiorum (CEN217) and S. rolfsii (CEN216), with nine isolates of Trichoderma. The clashes analyzed were: CEN217 x Trichoderma sp. (isolate CEN129); CEN217 x Trichoderma sp. (isolate CEN155); CEN217 x Trichoderma sp. (isolate CEN201); CEN217 x T. asperellum (isolate CEN1075); CEN216 x Trichoderma sp. (isolate CEN129); CEN216 x Trichoderma sp. (isolate CEN155); CEN216 x T. afroharzianum (isolate CEN281); CEN216 x T. afroharzianum (isolate CEN287); CEN216 x Trichoderma sp. (isolate CEN289); CEN216 x T. brevicompactum (isolate CEN1071) and CEN216 x Trichoderma sp. (isolate CEN1073).
The SEM showed the parallel growth and intertwining of hyphae of the pathogens and antagonists (Figs. 3 and 4), hydrolysis and deformation of the hyphae of the pathogens by the antagonists, as well as the penetration of Trichoderma spp., proving the hyperparasitism exerted by different Trichoderma isolates on S. sclerotiorum and S. rolfsii.
SEM images. (A) – Rede arrow pointing to sclerotia of Sclerotinia sclerotiorum (CEN217); (B) – Arrow indicating deformed hyphae of the pathogen; (C) – Arrow indicating hypha of Sclerotium rolfsii (CEN216) being parasitized by Trichoderma sp. (CEN1073 isolate); (D) – Arrow indicating hyphal coiling of the isolate CEN287 (Trichoderma afroharzianum) in S. rolfsii; (E) – Arrows indicating hyphae with appressoria of isolate CEN129 (Trichoderma sp.) on CEN216 and (F) – Arrow indicating hypha penetration of isolate CEN129 (Trichoderma sp.) in hypha of S. rolfsii
SEM images. (A) – Rede arrow indicating hyphae of the Trichoderma sp. (CEN155) parasitizing Sclerotinia sclerotiorum (CEN216); (B) – Arrow indicating the growth of hyphae of T. asperellum (isolate CEN1075) parallel to hyphae of the same pathogen; (C) – view of the confrontation region between Trichoderma sp. (tangle of hyphae more compacted at the base of the image) S. sclerotiorum (tangle of hyphae looser in the upper area of the image); (D) – View of the confrontation region between CEN281(T. afroharzianum) at the base of the image and CEN216 (S. rolfsii) in the upper area of the image; (E) – Reproductive structures of Trichoderma (CEN201), with its ampuliform phialid conidiogenic cells and smooth unicellular conidia; (F) – Arrows indicating intercalated chlamydospores of T. afroharzianum (CEN287)
Volatile metabolites
Of the 34 Trichoderma isolates tested for volatile metabolites production, none showed an apparent influence on the growth of S. sclerotiorum.
On the other hand, the results obtained in the assays for the production of active volatile metabolites against S. rolfsii led to the formation of three inhibition groups, as shown in Table 4. The mean silhouette length was 0.59, which indicates a reasonable separation of the groups of isolates. In accordance with Table 4, for this experiment, the best results were achieved with the isolates from group 3, which comprises nine isolates: CEN126, CEN155, CEN170, CEN169, CEN129, CEN290, CEN219, CEN1075 and CEN273, followed by group 1 with 14 isolates: CEN141, CEN201, CEN161, CEN1074, CEN289, CEN1069, CEN288, CEN209, CEN281, CEN194, CEN1068, CEN1073, CEN1079 and CEN162 and group 2 formed by 11 isolates: CEN1072, CEN1076, CEN316, CEN223, CEN1078, CEN1080, CEN1071, CEN287, CEN1077, CEN1070 and CEN280; finally, group 4 was constituted by the control. All groups of Trichoderma isolates used in this assay differed significantly from the control in terms of reducing the mycelial growth of S. rolfsii by the production of volatile compounds. Colonies of the phytopathogen reached lower growth than the control, which reached 9.0 cm in diameter, meaning that the antagonist isolates under study produce volatile toxic compounds capable of inhibiting the mycelial growth of isolate CEN216 and this may be one of the mechanisms of control of this pathogen by the antagonist isolates.
Non-volatile metabolites
In the study of the effect of non-volatile metabolites on S. sclerotiorum, based on the silhouette method, two inhibition groups were formed, as shown in Table 5. The mean silhouette length was 0.87, which indicates a good separation of the groups of isolates. The best results were achieved with group 2 isolates, namely: CEN1070, CEN1071, CEN1072, CEN1073, CEN1074, CEN1075, CEN1076, CEN1077, CEN1078, CEN1079, CEN1080, CEN129, CEN155, CEN162, CEN201, CEN209, CEN219, CEN273, CEN280, CEN281, CEN287 and CEN289. On the other hand, the results obtained with the other isolates were equal to the control and were allocated to group 2, that is, they did not produce soluble metabolites with properties inhibitory to the pathogen S. sclerotiorum.
As for the effect of non-volatile metabolites on S. rolfsii, two inhibition groups were formed, as shown in Table 5. The mean silhouette length was 0.8, which indicates a good separation. Composing group 2, with the best results, the isolates appear: CEN1070, CEN1077, CEN1072, CEN1074, CEN1073, CEN1079, CEN1080, CEN1076, CEN1078, CEN1071 and CEN129. These metabolites caused reduced growth of colonies, which were less dense and with deformations. The other isolates make up group 1 and did not produce soluble metabolites with inhibitory action on the mycelial growth of the pathogen.
Suppression of tomato seedlings damping-off in greenhouse
The Trichoderma isolates showed to be very promising when evaluated regarding their control of damping-off of tomato seedlings, in greenhouse, with emphasis on the isolates of group 1: CEN126, CEN1070, CEN1080 and CEN281, according to the average silhouette length method (Table 2). These isolates also showed good results in non-volatile metabolites production tests.
Discussion
Based on the present study, fungi isolate with good antagonistic capacity to the phytopathogenic fungi S. sclerotiorum and S. rolfsii were selected in laboratory and greenhouse evaluations. Isolates CEN281 and CEN287, both belonging to the species T. afroharzianum were the ones that exhibited antagonism in these tests.
As in the present work, some studies describe Trichoderma spp. inhibiting in vitro mycelial growth of S. sclerotiorum and S. rolfsii. Other team members of this work also observed good levels of S. sclerotiorum inhibition in dual culture with different Trichoderma isolates (Marques et al. 2016, 2022b; Mesquita et al. 2017). Sumida et al. (2018) reported that T. asperelloides isolates exhibited inhibition ranging from 60 to 100% of S. sclerotiorum. The same was observed in a study by Akhtar and Javaid (2018), where T. harzianum inhibited the growth of S. rolfsii, with a significant difference when compared to T. pseudokoninjii and T. ressei. Studies carried out by Kamel et al. (2020) showed that a T. koningii isolate exhibited a high inhibitory effect on S. rolfsii. Therefore, the interaction Trichoderma x soil-borne fungi is variable with the species and isolated from the biocontrol agent and also with the phytopathogenic species.
In the dual culture, antagonistic interactions were observed, typically due to competition and hyperparasitism, which according to Papavizas (1985) evidences the interaction between these microorganisms. The observed alterations evidence the parasitism of Trichoderma on the studied pathogens, as suggested by Papavizas (1985). Corroborating what was reported in this work, studies by Troian et al. (2014) and Kamel et al. (2020) showed that Trichoderma spp. has high levels of gene expression when parasitizing S. sclerotiorum and S. rolfsii, in addition to a synergistic action with the production of enzymes that degrade the fungal cell wall. Enzymes produced by Trichoderma spp., such as chitinases and cellulases, are known to lyse or degrade the pathogen’s cell wall (Ghasemi et al. 2020). Louzada et al. (2009) emphasize that only part of the evaluated Trichoderma isolates hyperparasitized S. sclerotiorum, suggesting that other mechanisms could be involved in the antagonism in vitro.
The results obtained in this work agree with those obtained by different authors, indicating that fungi of the genus Trichoderma have the ability to produce volatile metabolites with an inhibitory effect on the mycelial growth of several other fungi in the laboratory (Dennis and Webster 1971b). Kushwaha et al. (2018) observed an intermediary inhibition of this pathogen by T. harzianum, T. viride and T. virens. Among the metabolites produced by this group of fungi, there are gases, such as ethylene and hydrogen cyanide (Campbell 1989), which interfere with microbial growth. These volatile metabolites have activity at low concentrations but are not considered antibiotics. Besides these, the VOCs (Volatile Organic Compounds) acetoin, 2-methyl-1-propanol, 3-methyl-1-butanol and 6-pentyl-2-pyrone were the most ambiguous and produced by T. atroviride, when confronted with Phytophthora infestans (Elsherbiny et al. 2020).
Some non-volatile compounds produced by Trichoderma spp. have already been identified, in the pioneering work of Weidling (1934), where antibiotics such as gliotoxin and viridine were described; subsequentlyDennis and Webster (1971a),b) demonstrated the ability of some Trichoderma isolates to produce volatile and non-volatile compounds with an inhibitory effect on the development of several fungi. Among the effects caused by antibiotics, the reduction or stoppage of the growth and sporulation of the phytopathogen, reduction of spore germination, hyphal deformation and hydrolysis deserve to be highlighted (Campbell 1989). More examples of metabolites produced by the antagonist are pyrones, acids, furans, and lipids (Leylaie and Zafari 2018). Studies performed by other team members showed that non-volatile metabolites from T. ganense was not thermolabile and was able to completely inhibit S. sclerotiorum (Louzada et al. 2016), just as T. brevicompactum produced non-volatile metabolites capable of inhibiting the growth of S. rolfsii and S. sclerotiorum, among other phytopathogenic fungi (Marques et al. 2018). Kushwaha et al. (2018) also found that isolates of T. harzianum, T. viride and T. virens produced non-volatile metabolites with inhibitory action on S. rolfsii. Marques et al. (2022b) observed inhibition of mycelial growth of S. sclerotiorum by non-volatile metabolites of T. afroharzianum of up to 51.19%.
These data obtained corroborate others existing in the literature. As an example, Islam et al. (2016) reported that the incidence of tomato collar rot caused by S. rolfsii was lower in the treatment with T. harzianum, when compared with T. virens and T. asperellum. Later, Suriyagamon et al. (2018) showed satisfactory results in the biocontrol of S. rolfsii also in tomato, with isolates of T. harzianum and T. konnigii, respectively. Also under greenhouse conditions, T. koningii, T. viride and T. harzianum showed the highest antagonistic effect against three isolates of S. rolfsii, according to Kamel et al. (2020), as well as T. asperellum reduced the severity of this disease when associated with ammonium nitrate (Blanco et al. 2021).
Thirteen new Trichoderma isolates were obtained in areas cultivated with tomato at Embrapa Hortaliças (CNPH), Brazil, and incorporated into the Collection of Fungi for Biological Control of Embrapa/CENARGEN, expanding its gene pool. A variation in in vitro antagonism was observed both between species and within species of the evaluated antagonist fungi. According to these tests, isolates CEN281 and CEN287, both belonging to the species T. afroharzianum, showed potential as biocontrol agents for the phytopathogenic fungi Sclerotinia sclerotiorum and Sclerotium rolfsii. The isolates studied do not have inhibitory action against S. sclerotiorum due to the production of volatile metabolites. However, it is concluded that all of them were able to inhibit the growth of S. rolfsii by the production of these compounds. Isolates CEN281, CEN1070 and CEN1080 should be used in field studies, as they have presented the best results in the control of damping-off of tomato seedlings, caused by S. rolfsii, in greenhouse.
References
Abdelkhalek A, Al-Askar AA, Arishi AA, Behiry SI (2022) Trichoderma hamatum strain Th23 promotes tomato growth and induces systemic resistance against Tobacco mosaic virus. J Fungi 8(3):228. https://doi.org/10.3390/jof8030228
Agrofit - Phytosanitary Pesticides System of the Ministry of Agriculture, Livestock and Supply of Brazil (2023) Search for formulated products. Available at: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 20 February 2023
Akhtar R, Javaid A (2018) Biological management of basal rot of onion by Trichoderma harzianum and Withania somnifera. Planta Daninha 36. https://doi.org/10.1590/S0100-83582018360100009. Article e018170507
Alves E (2004) Curso introdutório à microscopia eletrônica de varredura. UFLA/FAEPE, Lavras
Asad SA (2022) Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases - a review. Ecol Complex 49: Article 100978. https://doi.org/10.1016/j.ecocom.2021.100978
Bell DK Wellls HD, Markham CR (1982) in vitro antagonism of Trichoderma species against six fungal plant pathogens Phytopathology 72:379–382. https://doi.org/10.1094/phyto-72-379
Benítez T, Rincón AM, Limón MC, Codón AC (2004) Biocontrol mechanisms of Trichoderma strains. Int Microbiol 7(4):249–260. https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1139 67092004000400003
Blanco NHM, Barbosa DFR, Graichen FAS (2021) Antagonistic microorganisms and nitrogen fertilization in control of tomato Southern blight. Arq Inst Biol 88 Article e00502019. https://doi.org/10.1590/1808-1657000502019
Campbell R (1989) Biological control of microbial plant pathogens. Cambridge University Press Cambridge, UK. https://doi.org/10.1007/BF00024971
Carvalho MR, Silva LR, Muniz PHPC, Carvalho DDC, Mello SCM (2021) Characterization of novel brazilians rhizosphere soil Trichoderma to select effective biocontrol agents against Sclerotinia sclerotiorum on bean. J Sci Res Rep 27(10):38–52. https://doi.org/10.9734/jsrr/2021/v27i1030448
Dennis DC, Webster J (1971a) Antagonistic properties of species-groups of Trichoderma. III. Hyphal interactions. Trans Brit Mycol Soc 57:363–369. https://doi.org/10.1016/S0007-1536(71)80050-5
Dennis DC, Webster J (1971b) Antagonistic properties of species-groups of Trichoderma. I. Production of non-volatile antibiotic. Trans Brit Mycol Soc 57:25–39. https://doi.org/10.1016/S0007-1536(71)80078-5
Elsherbiny EA, Amin BH, Aleem B, Kingsley KL, Bennett JW (2020) Trichoderma volatile organic compounds as a biofumigation tool against late blight pathogen Phytophthora infestans in postharvest potato tubers. J Agric Food Chem 68(3):8163–8171. https://doi.org/10.1021/acs.jafc.0c03150
Ghasemi S, Safaie N, Shahbazi S, Shams-Bakhsh M, Askari H (2020) The role of cell wall degrading enzymes in antagonistic traits of Trichoderma virens against Rhizoctonia solani. Iran J Biotechnol 18(4). https://doi.org/10.30498%2FIJB.2020.2333. Article e2333
Islam M, Hossain DM, Nonaka M, Harada N (2016) Biological control of tomato collar rot induced by Sclerotium rolfsii using Trichoderma species isolated in Bangladesh. Arch Phytopathol Plant Prot 50:109–116. https://doi.org/10.1080/03235408.2016.1265243
Jaiswa AK, Khadk R (2020) Trichoderma metabolites: versatile weapons against plant pathogens. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier, Amsterdam, Netherlands. https://doi.org/10.1016/B978-0-12-821007-9.00008-5
Kamel SM, Farag FM, Arafa RA, Essa TA (2020) Bio-Control potentials of Trichoderma spp. against Sclerotium rolfsii the causative of root and crown rot in tomato, common bean and cabbage. Egypt J Phytopathol 48:122–136. https://doi.org/10.21608/ejp.2020.54217.1018
Knapp S, Peralta IE (2016) The tomato (Solanum lycopersicum L., Solanaceae) and its botanical relatives. In: Causse M, Giovannoni J, Bouzayen M, Zouine M (eds) The tomato genome. Compendium of plant genomes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-53389-5_2
Kushwaha SK, Kumar S, Chaudhary B (2018) Efficacy of Trichoderma against Sclerotium rolfsii causing collar rot disease of lentil under in vitro conditions. J Appl Nat Sci 10(1):307–312. https://doi.org/10.31018/jans.v10i1.1622
Leylaie S, Zafari D (2018) Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from vinca plants. Front Microbiol 9:1484. https://doi.org/10.3389/fmicb.2018.01484
Louzada GAS, Carvalho DDC, Mello SCM, Lobo Júnior M, Martins I, Braúna LM (2009) Antagonist potential of Trichoderma spp. from distinct agricultural ecosystems against Sclerotinia sclerotiorum and Fusarium solani. Biota Neotrop 9(3):145–149. https://doi.org/10.1590/S1676-06032009000300014
Louzada GAS, Barbosa HN, Carvalho DD, Martins I, Lobo Junior M, Mello SCM (2016) Relações entre testes com metabólitos e seleção de isolados de Trichoderma spp. antagônicos a Sclerotinia sclerotiorum. R Bras Bioci 14(1):9–14. https://www.seer.ufrgs.br/rbrasbioci/article/view/114700
Machado AS, Ponciano NJ, Souza PM, Gravina GA, Daher RF (2018) Costs, viability and risks of organic tomato production in a protected environment. Cienc Agron 49(4):584–591. https://doi.org/10.5935/1806-6690.20180066
Marques E, Martins I, Cunha MOC, Lima MA, Silva JBT, Silva JP, Mello SCM (2016) New isolates of Trichoderma antagonistic to Sclerotinia sclerotiorum. Biota Neotrop 16(3):Articlee20160218. https://doi.org/10.1590/1676-0611-BN-2016-0218
Marques E, Martins I, Mello SCM (2018) Antifungal potential of crude extracts of Trichoderma spp. Biota Neotrop 18(1). https://doi.org/10.1590/1676-0611-BN-2017-0418. Article e20170418
Marques E, Abreu VPD, Silva MR, Castro KHMD, Cenci CMLDS, Almeida AC, Cunha MGD (2022a) Antifungal potential of Trichoderma afroharzianum metabolites. Intl J Agric Biol 28:181–186. https://doi.org/10.1590/1676-0611-bn-2017-0418
Marques E, Oliveira DR, Santos FHC, Castro KHM, Silva MR, Abreu VP, Cunha MG (2022b) Antagonism and molecular identification of Trichoderma isolated from rhizosphere of medicinal plants. J Biol Control 36(1):07–16. https://doi.org/10.18311/jbc/2022/30065
Mesquita DCM, Ferreira FA, Martins I, Mello SCM, Carvalho DDC (2017) Antagonismo in vitro de Trichoderma spp. a Sclerotinia sclerotiorum do feijão comum. ACSA 13(1):1–4. https://doi.org/10.30969/acsa.v13i1.710
Nelson R (2020) International Plant Pathology: past and future contributions to global food security. Phytopatholgy 110:245–253. https://doi.org/10.1094/PHYTO-08-19-0300-IA
Papavizas GC (1985) Trichoderma and Gliocladium: biology, ecology, and potential for biocontrol. Annu Rev Phytopathol 23:23–54. https://doi.org/10.1146/annurev.py.23.090185.000323
Persoon CH (1794) Disposita methodica fungorum. Römer’s Neues Mag Bot 1:81–128
Rousseeuw PJ (1987) Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 20:53–65. https://doi.org/10.1016/0377-0427(87)90125-7
Serra IMRS, Silva GS (2005) Caracterização biológica e fisiológica de isolados de sclerotium rolfsii obtidos de pimentão no Estado do Maranhão. Fitopatol Bras 30:61–66. https://doi.org/10.1590/S0100-41582005000100010
Silva JBT, Marques E, Menezes JE, Silva JP, Mello SCM (2020a) Population density of Trichoderma fungi in natural environments and agrosystems of a Cerrado area. Biota Neotrop 20(4):e2020a1048. https://doi.org/10.1590/1676-0611-BN-2020a-1048
Silva LR, Valadares-Inglis MC, Moraes MCB, Magalhães DM, Sifuentes DN, Martins I, Mello SCM (2020b) Morphological and protein alterations in Sclerotinia sclerotiorum (Lib.) De Bary after exposure to volatile organic compounds of Trichoderma spp. Biol Control 147:1–7. https://doi.org/10.1016/j.biocontrol.2020b.104279
Silva LR, Valadares-Inglis MC, Peixoto GHS, Luccas BEG, Muniz PHPC, Magalhães DM, Moraes MCB, Mello SCM (2021) Volatile organic compounds emitted by Trichoderma azevedoi promote the growth of lettuce plants and delay the symptoms of white mold. Biol Control 152:1–10. https://doi.org/10.1016/j.biocontrol.2020.104447
Singh VK, Singh AK, Kumar A (2017) Disease management of tomato through PGPB: current trends and future perspective. Biotech 7(4):255. https://doi.org/10.1007/s13205-017-0896-1
Smolińska U, Kowalska B (2018) Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum – a review. J Plant Pathol 100:1–12. https://doi.org/10.1007/s42161-018-0023-0
Sumida CH, Daniel JFS, Araujod APCS, Peitl DC, Abreu LM, Dekker RFH, Canteri MG (2018) Trichoderma asperelloides antagonism to nine Sclerotinia sclerotiorum strains and biological control of white mold disease in soybean plants. Biocontrol Sci Technol 28(2):142–156. https://doi.org/10.1080/09583157.2018.1430743
Suriyagamon S, Phonkerd N, Bunyatratchata W, Riddech N, Mongkolthanaruk W (2018) Compost seed of Trichoderma harzianum UD12-102 in controlling collar and stem rot of tomato caused by Sclerotium rolfsii. Environ Nat Resour J 16(2):20–28. https://doi.org/10.14456/ennrj.2018.12
Thambugala KM, Daranagama DA, Phillips AJL, Kannangara SD, Promputtha I (2020) Fungi vs. fungi in biocontrol: an overview of fungal antagonists applied against fungal plant pathogens. Front Cell Infect Microbiol 10:604923. https://doi.org/10.3389/fcimb.2020.604923
Troian RF, Steindorff AS, Ramada MH, Arruda W, Ulhoa CJ (2014) Mycoparasitism studies of Trichoderma harzianum against Sclerotinia sclerotiorum: evaluation of antagonism and expression of cell wall-degrading enzymes genes. Biotechnol Lett 36(10):2095–2101. https://doi.org/10.1007/s10529-014-1583-5
Weidling R (1934) Studies on a lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopathology 24:1153–1179
Zubrod JP, Bundschuh M, Arts G (2019) Fungicides: an overlooked Pesticide Class? Environ Sci Technol 53(7):3347–3365. https://doi.org/10.1021/acs.est.8b04392. Bruhl CA, Imfeld G, Knabel A, Payraudeau S, Rasmussen JJ, Rohr J, Schamuller A, Smalling K, Stehle OS, Schulz R, Scafer RB
Acknowledgements
The authors are grateful for the scholarships granted by CNPq and CAPES, as well as financial support for the research project guaranteed by FAP-DF.
Author information
Authors and Affiliations
Contributions
Conceptualization and Investigation: [Sueli Correa Marques de Mello]; Formal Analysis: [Sandro Coelho Linhares Montalvão] and [Eder Marques] and [Irene Martins]; Data curation: [Joseane Padilha da Silva]; Supervision: [Sueli Correa Marques de Mello]; Writing [Sandro Coelho Linhares Montalvão] and [Eder Marques].
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Montalvão, S.C.L., Marques, E., Martins, I. et al. Suppression of the phytopathogens Sclerotinia sclerotiorum and Sclerotium rolfsii by Trichoderma spp.. Biologia 78, 2941–2952 (2023). https://doi.org/10.1007/s11756-023-01457-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01457-9