Abstract
Purpose
The growth behavior of novel fungal isolates along with the production of bioactive compounds from mycelia mass during submerged fermentations were investigated using conventional synthetic media and agro-industrial residues as fermentation substrates.
Methods
Four novel isolated fungi, belonging to edible or medicinal species, were evaluated for their ex-situ growth on potato dextrose agar. Specific attention was designated to Sepedonium sp. and Phellinus sp. considering their high growth rate in solid state fermentations. Submerged fermentations were subsequently employed using synthetic carbon sources (glucose, fructose and lactose) to assess the fermentation behavior. Following the better growth pattern on glucose and fructose, compared to lactose, grape pomace extract (GPE) was applied as nutrient feedstock to assess the production of biomass and bioactive compounds. Aqueous extraction was performed to obtain crude intracellular polysaccharides (IPS), that were subsequently characterized in terms of antioxidant activity, protein and polysaccharide content.
Results
Sepedonium sp. demonstrated the highest biomass production; 11.4 and 10.5 g/L, using glucose and fructose, respectively, whereas Phellinus yielded up to 3.8 g/L. Lactose was also assimilated by both fungal strains, however biomass production was lower. Utilization of GPE affected biomass production; Sepedonium sp. biomass decreased, whereas biomass obtained from Phellinus sp. was enhanced, compared to synthetic sugars. Crude IPS extracts elicit high antioxidant activity (> 75% inhibition of DPPH• free radical).
Conclusion
The successful application of conventional and renewable substrates for Sepedonium and Phellinus fermentation was demonstrated, while the mycelia mass derived polysaccharide-protein complexes exhibited bioactive properties, and thus might be utilized as functional food components.
Graphical Abstract

Similar content being viewed by others
Statement of Novelty
In this study four indigenous fungal isolates were investigated, while the initial results indicated the potential of two specific species, namely Sepedonium sp. and Phellinus sp. These have been reported as potential producers of bioactive compounds, however information regarding their fermentation behavior is scarce in the open literature. In addition, the majority of research concerning the production of bioactive extracts has been performed on fruiting bodies and rarely on the mycelium deriving from synthetic carbon sources. In line with the above, and considering that current development of novel products should coincide with the bio-economy strategy, this study evidenced that the aforementioned species had the ability to grow on grape pomace extract and produce polysaccharide-protein complexes with antioxidant properties. Our findings revealed a profound perspective for further investigation of unexplored fungal species towards the production of bioactive components of nutraceutical and pharmaceutical interest.
Introduction
A recent commentary highlighted the importance of novel food innovations and the key role of functional foods to actualize Sustainable Development Goals (SDG) target 2 encompassing food security and improved nutrition, promoting sustainable agriculture as promulgated by the UN [1]. The “nutrition transition” includes healthier dietary changes to prevent chronic disease and promote human health [2]. Equally, the deployment of traditional foods—often disregarded with the development of economic wealth—could complement the resilience of food systems.
The interest to promote human wellbeing is evidenced by the flourishing nutraceutical and dietary supplements market, with a Compound Annual Growth Rate (CAGR) of 7.5% by 2025 [3]. A recent review by Niego et al. [3], highlighted that the market for edible mushrooms is expected to amount to US$ 72.5 billion by 2027, whereas, the medicinal mushroom market is estimated to amount to US$ 13.88 billion by 2022 indicating a CAGR of 9.15% [3].
Medicinal mushrooms (fungi) including species of Trametes, Ganoderma, Pleurotus, Lentinula and Phellinus species have been traditionally used for their beneficial health properties, mainly in countries of East Asia [4, 5]. Approximately 32.5% of the edible mushrooms (650 out of 2000 safe species) have evidenced medicinal attributes via the action of bioactive compounds, including coriolan, ganoderiol, lentinan, schizophyllan, hispidin among others [6,7,8]. Health benefits include anti-diabetic, immunomodulatory, antioxidant, antimicrobial, anticancer and prebiotic properties, indicated by in vitro tests and clinical trials [5, 6]. Mushroom polysaccharides and polysaccharides-protein complexes confer compounds of vital interest, with established bioactive properties, whereas inclusion in functional foods has been also referred [5, 9]. Polysaccharides are distinguished as extracellular (exopolysaccharides, EPS) or intracellular (IPS), and can be obtained from the fruiting bodies, mycelia mass and the culture broth. Several processing methods have been employed to isolate fungal polysaccharides, still hot water extraction suggests the most beneficial and frequently applied method [5]. On top of that, the polysaccharide complexes elicit antioxidant activity, dependent on the tertiary structure, conformation, purification level, water solubility etc. [10]. The majority of the studies investigating the bioactive properties of such compounds often undertake the implementation of fruiting bodies as opposed to the mycelia mass, considering that hot water extracts have been also commercialized [11]. Nonetheless, controlled fermentation of fungal strains with specific focus on medicinal mushrooms, has been successfully performed either in submerged or in solid state to generate several metabolites [10, 12, 13]. To further advocate the sustainability and the economic feasibility of such processes, that will subsequently conform with the bio-economy concept, various agro-industrial waste and by-products streams have been examined as fungal fermentation feedstocks [14,15,16].
The genus Phellinus is a medicinal mushroom, belonging to Basidiomycetes, and comprises several species (e.g., P. linteus, P. ingiarius, P. baumii) with previously established medicinal attributes [5, 10, 17, 18]. However, the natural field-cultivation of Phellinus is scarce and requires significant amount of time, hence previous studies highlighted the prominence of submerged fermentation as an emerging alternative [10, 18]. On the other hand, the filamentous fungi Sepedonium is a mycoparasitic genus, often infecting the basidiocarps of Boletales [19, 20]. Fungal strains of Sepedonium genus have been examined for antibiotic and pigment production with potential pharmaceutical applications, with specific focus on peptaibols-peptides of diversified structure and bioactivity [21].
Thereof, to advance sustainable food systems, research should be directed towards the valorization of agro-industrial streams and generate products with bioactive properties, included in the concept of “nutrition transition” for enhanced human health. Likewise, the controlled cultivation of medicinal mushrooms with specific focus on polysaccharide-protein complexes is unequivocally of utmost importance. Hence, the aim of this study was to assess the growth behavior and the production of bioactive compounds by two novel indigenous fungal isolates, namely, Sepedonium sp. and Phellinus sp. Synthetic media with conventional carbon sources but also agro-industrial residues, specifically grape pomace, were evaluated as substrates during submerged fermentations. The ability to produce bioactive compounds was also evaluated, with specific focus on crude IPS and their antioxidant activity, along with their preliminary chemical characterization. In fact, this study elaborates on the prominent potential of utilizing the mycelia mass to extract bioactive compounds using submerged fermentation on agri-residues, particularly on grape pomace, in the context of sustainable bioprocesses to conform with the principles of bio-economy.
Materials and Methods
Biological Material and Culture Conditions
Three indigenous mushrooms, namely Phellinus sp., Macrolepiota procera and Oudemanciella melanotricha, and a microfungi strain, namely Sepedonium sp. were used in the current study (Fig. 1). All fungal strains were isolated from Kefalonia island and deposited in the fungal culture collection of the Laboratory of Food Chemistry and Industrial Fermentations of the Department of Food Science and Technology at the Ionian University (Greece). The strains were maintained in inclined potato dextrose agar (PDA) slants, filled with paraffin oil at 5.0 ± 1.0 °C, and also at − 20.0 ± 1.0 °C in 20% (w/v) glycerol. PDA plates were used for the resuscitation of the fungal strains. Subsequently, agar plugs (6 mm diameter) from PDA plates were used to prepare fresh mycelium to be used as inocula for the submerged fermentations in a liquid preculture. The liquid precultures were prepared in Erlenmeyer flasks (500 mL) filled with 150 mL of a synthetic glucose-based medium (pH 6.2) consisting of various nutrients and elements, as previously described [12]. The flasks were sterilized for 20 min at 121 ± 1 °C and then inoculated. All aforementioned incubations were performed at 25.0 ± 0.5 °C at the designated time for each strain, whereas liquid precultures were incubated under agitated conditions (150 rpm).
Solid State Fermentations (SSF) to Estimate Growth Rate
SSF were performed on PDA plates (90 mm diameter) targeting to assess the growth rate of all four fungal strains. In particular, a mycelium agar plug (6 mm diameter) was obtained from a freshly prepared growing colony and aseptically placed at the center of a PDA plate (pH 6.2), followed by incubation at 25 ± 0.5 °C. For each strain, at least three replicates were performed to study the growth kinetics with respect to the radius mycelium growth rate, as described in a following section.
Submerged Fermentations (SmF)
Initial submerged fermentations were conducted on synthetic media with different commercial carbon sources, namely glucose, fructose and lactose at a starting concentration of ~ 12 g/L. The composition of the synthetic media was as follows (in g/L): yeast extract 2.5; peptone 3.5; CaCO3 2.0; KH2PO4 1.0; MgSO4·7H2O 0.5; CaCl2·2H2O 0.23; MnSO4·H2O 0.04; ZnSO4·7H2O 0.02; FeCl3·6H2O 0.08 and the pH was adjusted at 6.2. Subsequently, SmF were conducted using as the sole fermentation supplement the aqueous extract of grape pomace (GPE). More specifically, grape pomace was derived from a local vineyard, as a side stream of the vinification process of the red variety Mavrodaphni (Kefalonia, Greece), and included skins and seeds. The extraction of free sugars was performed at 50 °C for 1 h, at a solid-to-liquid ratio of 1:4 (w/v) with deionized water [22]. The initial composition of GPE contained equal quantities of glucose and fructose and minor quantities of sucrose (approximately 10 g/L total sugars). For the SmF, 45 mL of each medium was added in Erlenmeyer flasks (200 mL), the pH was adjusted at 6.2, autoclaved (121 ± 1 °C, 20 min) and inoculated with the preculture (10%, v/v). Incubations were conducted in static conditions and duplicate samples were collected at specific time intervals to monitor fungal growth and sugar consumption. Fermentations were monitored up to 13 days.
Analytical Methods
Mycelium Growth Rate
The radius growth rate (Kr, mm/day) of the fungal strains was evaluated by measuring the colony diameter on the surface of SSF. Measurements were monitored in two perpendicular directions every 24 h, until the Petri dish was completely colonized by the fungus.
Total Dry Weight (TDW)
TDW (g/L) was determined gravimetrically. First, the fungal biomass was separated from the culture broth by filtration (Whatman No 1, England) under vacuum, washed twice with deionized water and dried in an oven at 60 ± 0.5 °C until a constant weight was obtained. The clear filtrate was employed for the determination of residual sugars. Dry biomass was also used for further analysis to assess the composition of the fungal mycelia.
Total Phenolic Content (TPC) and Free Amino Nitrogen (FAN)
Total phenolic content (TPC) of dried grape pomace was performed using aqueous ethanol solution (70%) following the protocol of our previous study [23]. A solid-to-solvent ratio of 1 to 40 was utilized and the extraction process was carried out in an ultrasonic bath (40 kHz, 130 W, Sonica 2400 M S3, Soltec, Milano, Italy) for 20 min. The resulting extract was evaporated using a vacuum rotary evaporator (40 °C), and the dry extract was re-dissolved in 5 mL methanol. The methanolic extracts were stored at − 20 °C, until their further characterization. TPC results were expressed as gallic acid equivalents. Free amino nitrogen (FAN) concentration was measured following a previously described method [24].
Preparation of Extract
Hot water extraction was performed on the mycelial biomass to assess the protein content, total sugars and antioxidant activity following a method previously described [4, 25]. Briefly, a sample of dried mass was extracted by stirring with boiling water at 100 °C at a solid-to-liquid ratio (1:10, w/v) for 1 h. The liquid residue was filtered and freeze dried. The dried extract was resuspended in deionized water (20 mg/mL) and stored at 4 °C prior to further analysis. Aqueous extraction was selected on the basis of future implementation of the extract in food formulation.
Sugar Analysis and Protein Content
Glucose, fructose and lactose were determined using High Performance Liquid Chromatography analysis (HPLC, Agilent), equipped with a ROA-organic acid H + (300 mm × 7.8 mm, Phenomenex) column coupled to a differential refractometer (RID). Operating conditions were as follows: sample volume 10 μL; mobile phase 10 mM H2SO4; flow rate 0.6 mL/min; column temperature 65 °C. Samples were diluted and filtered (Whatman®, 0.2 μm) prior to analysis.
Characterization of the Aqueous Extract
Polysaccharides content in the extract were determined via the phenol–sulfuric method using glucose as the standard [26]. The protein content was determined using the Lowry method [27]. The antioxidant activity was evaluated using the DPPH· (2,2-diphenyl-1-picrylhydrazyl) scavenging radical method. The percent inhibition (I%) of the free radical was estimated using the following equation:
where ABSDPPH· was the absorbance of the blank and ABSsample was the absorbance of the sample.
Antioxidant activity was also expressed as μg of Trolox equivalents per mg of dry fungal extract.
Thin layer chromatography (TLC) method was performed as a preliminary tool for amino acids composition using pre-developed plates (TLC Silica gel 60 F254, Sigma Aldrich) in chloroform: methanol (2:1 v/v). The samples (5 μL) were developed using chloroform: methanol: glacial acetic acid: water (25:15:4:2, v/v) as a solvent system. Dry plates were sprayed with a solution of 0.2% (w/v) ninhydrin in ethanol and placed in an oven at 100 °C for 15 min to visualize amino acid bands.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel 2018, and values are presented as average ± standard deviation.
Results and Discussion
Mycelium Growth Rate
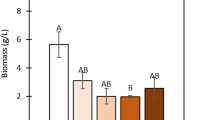
Initial experiments targeted the investigation of the mycelium growth rate in SSF using PDA, as a conventional and beneficial substrate for fungal strains. Figure 2 illustrates the average radius growth rate (mm/day) for all strains. It can be easily observed that Sepedonium sp. and Phellinus sp. demonstrated the maximum linear growth, i.e., ~ 6.71 and ~ 5.92 mm/day respectively, coinciding with previous studies in the open literature. For instance, the diversity of 35 mycoparasite Sepedonium strains was recently studied and the authors reported an in vitro growth rate ranging from 4.6 to 7.5 mm/day [20]. The effect of culture conditions on the mycelial growth for three Phellinus spp. has been also undertaken [28], whereby the growth was found 7.63 mm/day and 6.36 mm/day (68.7 and 57.3 mm/9 days) for P. baummi and P. gilvus respectively using PDA as the substrate. Similarly, PDA performed better among different substrates tested for several Phellinus strains, indicating an average value of 20.8 mm/10 days [29]. The remaining strains demonstrated low linear growth, hence Sepedonium sp. and Phellinus sp. were selected for further investigation.
SmF Using Conventional Carbon Sources
Following the results of the linear growth rate, Sepedonium sp. and Phellinus sp. were evaluated in SmF cultures using conventional synthetic media and different carbon sources (Fig. 3). Glucose, fructose and lactose were employed to investigate the growth performance, in view of substituting these commercial carbohydrate sources with nutrients obtained from agro-industrial waste and by-products. Fermentations were monitored up to 6 days, where the carbon source was depleted, based on preliminary experiments (data not shown). As observed in Table 1, Sepedonium sp. demonstrated the highest biomass using glucose and fructose (11.4 and 10.5 g/L respectively), whereas lactose entailed 3.2 g/L of TDW. Likewise, the highest biomass productivity (Qx) was obtained for glucose and fructose. Similarly, Phellinus sp. performed better on glucose and fructose, however a lower range of biomass production was obtained, yielding maximum 3.8 g/L in glucose. Evidently, the results obtained for both strains were lower when lactose was implemented. In fact, lactose consumption correlates to the amount and activity of the enzyme β-galactosidase, that is regulated by the Lac operon. When lactose is used as the substrate, a part of the available lactose (approximately 50%) will be transglycosylated to allolactose to activate the Lac operon and produce β-galactosidase. The remaining lactose and part of allolactose, will be hydrolyzed to generate glucose and galactose. These three enzymatic functions are regulated by β-galactosidase. The binding of allolactose in lacZ repressor creates a positive feedback loop which regulates the amount of β-galactosidase in the cell. Hence, compared to the metabolic pathway of glucose, the process of lactose hydrolysis and consumption demonstrates a more complicated and potentially more “energy requiring” process [30].
Previous studies have undertaken the study of Phellinus sp. via fermentation on conventional substrates, in an attempt to achieve controlled cultivation to obtain target compounds for medicinal use [18]. Likewise, various carbon and nitrogen sources were employed to evaluate the growth and polysaccharides production of P. linteus in submerged fermentations [31]. The authors noted that the optimal medium included fructose as the carbon source (40 g/L), yielding 29.9 g/L of biomass in a jar fermentor after 7 days. In another study, experimental design indicated glucose as the optimal carbon source for P. nigricans, targeting exopolysaccharides production [32]. Cheese whey permeate was evaluated for SSF and SmF of P. linteus mycelium using response surface methodology [33]. In the case of submerged cultures, a growth rate of 192.1 mg/L per day was reported at an initial lactose concentration of 65.3 g/L [33], that is lower to the one obtained in this study, yet highlights the potential to implement cheese whey as fermentation supplement. On the other hand, controlled submerged fermentations of Sepedonium strains are quite scare in the open literature, focusing mainly on pigment synthesis, the antibiotic activity of sepedonin, or the antagonistic effect against pathogens [34]. For instance, Nagao et al. studied sepedonin production during fermentation of S. chrysospermum NT-1 on CY-1 glucose based medium, to further investigate the antimicrobial effect on several bacteria, yeasts and molds [35]. Sophisticated analysis on the structure of secondary metabolites of Sepedonium have been evidenced [19, 21]; still, to realize the methodical production of these compounds (with biopharmaceutical interest), it is crucial to first consider bioprocess optimization through the use of diversified carbon substrates, including C5, C6 sugars or oligosaccharides.
Evaluation of GPE as Nutrient Feedstock for SmF
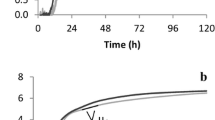
Based on the results obtained from commercial carbon sources, our study proceeded with the substitution of conventional media with nutrient supplements obtained from renewable resources, specifically grape pomace, as a by-product of wine making. Initially, a preliminary characterization of GPE was performed to determine the TPC. TPC of “Mavrodaphni” variety grape pomace was determined 0.55 g/100 g dry weight. Generally, TPC of grape pomace have been reported in the range of 0.28–8.7 g/100 g grape pomace, depending on the variety, consisting mainly of anthocyanins, catechins, flavonol glycosides and phenolic acids among others [36]. As described earlier, GPE was evaluated in SmF during static conditions, using both fungal strains, and the results are shown in Fig. 3 for Sepedonium sp. and Fig. 4 for Phellinus sp. In this case, fermentations were monitored up to 13 days and 9 days for Sepedonium sp. and Phellinus sp., respectively. TDW production of Sepedonium sp. decreased significantly using GPE compared to pure glucose (6.8 g/L and 11.4 g/L, respectively), resulting in a proportional reduction of biomass productivity (from 1.82 to 0.72 g/L/d). Still it is worth noting that fungal growth was not inhibited by the substrate, considering also that GPE was not supplemented with exogenous nitrogen sources. Fructose was not totally consumed whereas in the case of commercial fructose, complete consumption was obtained.
On the other hand, biomass production of Phellinus sp, was enhanced when GPE was applied as nutrient feedstock. TDW increased from 3.8 and 3.2 g/L (glucose and fructose, respectively) to 4.9 g/L. Fructose consumption was also improved; in the case of commercial fructose the strain consumed ~ 46% of the initial fructose, whereas in the case of GPE fructose consumption reached ~ 54%. Thus, the effective utilization of GPE was evidenced for this strain also. Substrate to biomass conversion yield (YX/S) was estimated at 0.87 g/g and 0.59 g/g for Sepedonium and Phellinus sp. respectively. Previous studies in the literature have indicated a wide range for YX/S coefficient values, susceptible to the strain and the substrate employed [37, 38]. It should be noted that GPE contained low amount of free amino nitrogen (30–50 mg/L) that was consumed during the fermentation process. It is possible that the addition of yeast extract or peptone could further improve biomass production (to be comparable with the synthetic media) along with the effect of temperature, pH and agitation but this will be elaborated in future studies. Likewise, the presence of high TPC (602.8 mg/L) in the fermentation feedstock may impair on the fungal metabolism; however the obtained results do not indicate significant inhibitions. An attempt to assess the consumption of phenolics during fermentation was performed but the simultaneous production of several metabolic products (e.g., biocolorants) could compromise the results obtained using the Folin method (data not shown). Nonetheless, the bioprocess for the controlled fermentation of the aforementioned fungal strains indicates the potential to be integrated in an existing vinification facility and mediate the management of wine making by-product streams via the proposed biotechnological route. Evidently, a techno-economic evaluation and an environmental impact assessment should corroborate on the sustainability of the process.
Characterization of the Fungal Mycelia Extracts
The ultimate part of the current study entailed a preliminary characterization of the crude IPS obtained from fungal biomass, at the point where maximum biomass was achieved using GPE (Table 2). A preliminary material balance indicated that processing 1 kg of grape pomace could entail the production of 4.36 g/L and 14.68 g/L crude IPS using Phellinus and Sepedonium strains, respectively (based on the extraction yield). The protein content in the extract of Sepedonium sp. was 19.2%, in line with previous studies [12], whereas the polysaccharide content was around 29.2%. The aqueous extract exhibited significant antioxidant activity (I% = 78), evidenced by the percentage of inhibition of the free DPPH· radical. The antioxidant activity was also expressed as 13.8 μg Trolox/mg extract. Similarly, Phellinus sp. strain demonstrated adequate protein content, equal to 26.1%, hence postulating its significance as functional component. In fact, mushrooms contain significant amount of protein, amino acids and bioactive polysaccharides, heteroglucans, peptidoglucans, proteoglucans etc. [4, 26]. In a recent study, Lung and Deng, refered to the extraction of EPS and IPS from the mycelia mass of P. igniarius after SmF in stirrred bioreactor [26]. Therein authors reported a high protein/polysaccharide ratio (3.02–3.68%). In our study, the polysaccharides content was 16.9%, hence a protein/polysaccharides ratio of 1.54 (%, w/w) can be estimated. Nonetheless, in our study we performed a preliminary characterization to identify the potential of bioactive compounds, considering the rising interest for EPS, IPS and antioxidants from fungal strains with medicinal properties. Likewise, the extract demonstrated antioxidant activity, based on the reduction of the DPPH· free radical (I% = 75%). In fact, the antioxidant activity of extracts obtained from the fruiting bodies, but also the mycelia mass, have been previously reported [5]; however a precise comparison is hindered as it relies on the scavenging method applied during the experimental set up. Results of TLC analysis indicated the presence of lysine, aspartic acid, cysteine, leucine and glycine (Fig. 5), still an in-depth analysis of the composition of polysaccharide-protein complexes is necessary, which is actually the subject of our ongoing research. Previous studies on the composition of Phellinus polysaccharides have indicated glucose, mannose, galactose, xylose, arabinose rhamnose and fucose as the principal constituents [18, 32, 39]. In fact, Phellinus polysaccharides confer bioactive compounds of paramount importance and could elicit benefits for the human health or applied for functional foods development.
Technological Significance and Future Perspectives
In the context of transitioning to circular economy, the design of bioprocesses should address the preservation of nutrient circularity. The results of our study demonstrate the potential to implement the nutrients contained in grape pomace and generate a fermentation supplement for the controlled cultivation of mycelium of medicinal fungi. Likewise, future studies could allocate a twofold approach. First the assessment of other agricultural and food manufacturing side streams should be considered. For instance citrus peel or bakery waste have previously evidenced the ability to provide essential nutrients for fungal growth [40, 41]. In a similar concept, Sepedonium strain could be evaluated for colorant production, given the emerging interest and the potential use in medical application. Secondly, more elaborative extraction and sophisticated purification steps should be employed, to isolate both intracellular and extracellular polysaccharides. Likewise, the biological activities should be elucidated to determine the potential application in the pharmaceutical and/or food industries.
Conclusions
The results of this study demonstrate the growth pattern and the fermentation behavior of two novel fungal isolates, namely Sepedoniun and Phellinus, using conventional as well as renewable substrates. The implementation of GPE as a renewable fermentation supplement did not inhibit fungal proliferation and the production of bioactive compounds. On top of that, the successful extraction of crude IPS from mycelia mass with bioactive attributes is advocated. Evidently, the outcomes of this study contribute on the restricted current knowledge about controlled fermentation of medicinal fungi Sepedoniun and Phellinus, and highlight the emerging potential to exploit and valorize agro-industrial waste and by-products towards the production of bioactive molecules envisaging their incorporation in functional foods to increase the circularity of the bioprocess.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Mensi, A., Udenigwe, C.C.: Emerging and practical food innovations for achieving the Sustainable Development Goals (SDG) target 2.2. Trends Food Sci. Technol. 111, 783–789 (2021). https://doi.org/10.1016/j.tifs.2021.01.079
Sammugam, L., Pasupuleti, V.R.: Balanced diets in food systems: emerging trends and challenges for human health. Crit. Rev. Food Sci. Nutr. 59, 2746–2759 (2019). https://doi.org/10.1080/10408398.2018.1468729
Niego, A.G., Rapior, S., Thongklang, N., Raspé, O., Jaidee, W., Lumyong, S., Hyde, K.D.: Macrofungi as a nutraceutical source: promising bioactive compounds and market value. J. Fungi 7, 397 (2021)
Chun, S., Gopal, J., Muthu, M.: Antioxidant activity of mushroom extracts/polysaccharides: their antiviral properties and plausible antiCOVID-19 properties. Antioxidants 10, 1899 (2021)
Yan, J.K., Pei, J.J., Ma, H.L., Wang, Z.B., Liu, Y.S.: Advances in antitumor polysaccharides from phellinus sensu lato: production, isolation, structure, antitumor activity, and mechanisms. Crit. Rev. Food Sci. Nutr. 57, 1256–1269 (2017). https://doi.org/10.1080/10408398.2014.984802
Panda, S.K., Luyten, W.: Medicinal mushrooms: clinical perspective and challenges. Drug Discov. Today. 27, 636–651 (2022). https://doi.org/10.1016/j.drudis.2021.11.017
Giavasis, I.: Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 26, 162–173 (2014). https://doi.org/10.1016/j.copbio.2014.01.010
Hyde, K.D., Xu, J., Rapior, S., Jeewon, R., Lumyong, S., Niego, A.G.T., Abeywickrama, P.D., Aluthmuhandiram, J.V.S., Brahamanage, R.S., Brooks, S., Chaiyasen, A., Chethana, K.W.T., Chomnunti, P., Chepkirui, C., Chuankid, B., de Silva, N.I., Doilom, M., Faulds, C., Gentekaki, E., Gopalan, V., Kakumyan, P., Harishchandra, D., Hemachandran, H., Hongsanan, S., Karunarathna, A., Karunarathna, S.C., Khan, S., Kumla, J., Jayawardena, R.S., Liu, J.-K., Liu, N., Luangharn, T., Macabeo, A.P.G., Marasinghe, D.S., Meeks, D., Mortimer, P.E., Mueller, P., Nadir, S., Nataraja, K.N., Nontachaiyapoom, S., O’Brien, M., Penkhrue, W., Phukhamsakda, C., Ramanan, U.S., Rathnayaka, A.R., Sadaba, R.B., Sandargo, B., Samarakoon, B.C., Tennakoon, D.S., Siva, R., Sriprom, W., Suryanarayanan, T.S., Sujarit, K., Suwannarach, N., Suwunwong, T., Thongbai, B., Thongklang, N., Wei, D., Wijesinghe, S.N., Winiski, J., Yan, J., Yasanthika, E., Stadler, M.: The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 97, 1–136 (2019). https://doi.org/10.1007/s13225-019-00430-9
Chaturvedi, V.K., Agarwal, S., Gupta, K.K., Ramteke, P.W., Singh, M.P.: Medicinal mushroom: boon for therapeutic applications. 3 Biotech 8, 334 (2018). https://doi.org/10.1007/s13205-018-1358-0
Zhang, H.-N., Ma, H.-L., Zhou, C.-S., Yan, Y., Yin, X.-L., Yan, J.-K.: Enhanced production and antioxidant activity of endo-polysaccharides from Phellinus igniarius mutants screened by low power He-Ne laser and ultraviolet induction. Bioact. Carbohydr. Diet Fibre 15, 30–36 (2018). https://doi.org/10.1016/j.bcdf.2016.11.006
Wu, J.-Y.: Polysaccharide-protein complexes from edible fungi and applications. In: Ramawat, K.G., Mérillon, J.-M. (eds.) Polysaccharides, pp. 1–10. Springer, Cham (2014)
Papadaki, A., Lappa, I.K., Kachrimanidou, V., Gonou-Zagou, Z., Kopsahelis, N.: Trametes versicolor as a natural source of bioactive compounds for the production of whey protein films with functional properties: a holistic approach to valorize cheese whey. Waste Biomass Valoriz. 13, 3989–3998 (2022). https://doi.org/10.1007/s12649-022-01874-y
Diamantis, I., Melanouri, E.M., Dedousi, M., Panagopoulou, I., Papanikolaou, S., Stoforos, N.G., Diamantopoulou, P.: Sustainable and eco-friendly conversions of olive mill wastewater-based media by Pleurotus pulmonarius cultures. Fermentation 8, 129 (2022). https://doi.org/10.3390/fermentation8030129
Papadaki, A., Kachrimanidou, V., Papanikolaou, S., Philippoussis, A., Diamantopoulou, P.: Upgrading grape pomace through Pleurotus spp. Cultivation for the production of enzymes and fruiting bodies. Microorganisms 7, 207 (2019). https://doi.org/10.3390/microorganisms7070207
Papadaki, A., Diamantopoulou, P., Papanikolaou, S., Philippoussis, A.: Evaluation of biomass and chitin production of morchella mushrooms grown on starch-based substrates. Foods 8, 239 (2019). https://doi.org/10.3390/foods8070239
Meini, M.-R., Ricardi, L.L., Romanini, D.: Novel routes for valorisation of grape pomace through the production of bioactives by Aspergillus niger. Waste Biomass Valoriz. 11, 6047–6055 (2020). https://doi.org/10.1007/s12649-019-00844-1
Yang, K., Zhang, S., Ying, Y., Li, Y., Cai, M., Guan, R., Hu, J., Sun, P.: Cultivated fruit body of Phellinus baumii: a potentially sustainable antidiabetic resource. ACS Omega 5, 8596–8604 (2020). https://doi.org/10.1021/acsomega.9b04478
Chen, H., Tian, T., Miao, H., Zhao, Y.-Y.: Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: a review. Fitoterapia 113, 6–26 (2016). https://doi.org/10.1016/j.fitote.2016.06.009
Lam, Y.T.H., Ricardo, M.G., Rennert, R., Frolov, A., Porzel, A., Brandt, W., Stark, P., Westermann, B., Arnold, N.: Rare glutamic acid methyl ester peptaibols from Sepedonium ampullosporum damon KSH 534 exhibit promising antifungal and anticancer activity. Int. J. Mol. Sci. 22, 12718 (2021)
Binimelis-Salazar, J., Casanova-Katny, A., Arnold, N., Lima, C.A., Norambuena, H.V., González-Rocha, G., Palfner, G.: Diversity and host relationships of the mycoparasite sepedonium (Hypocreales, Ascomycota) in temperate central Chile. Microorganism 9, 2261 (2021)
Vargas, D.F., Larghi, E.L., Kaufman, T.S.: First total synthesis of ampullosine, a unique isoquinoline alkaloid isolated from Sepedonium ampullosporum, and of the related permethylampullosine. RSC Adv. 9, 33096–33106 (2019). https://doi.org/10.1039/C9RA06839B
Tsouko, E., Papadaki, A., Papapostolou, H., Ladakis, D., Natsia, A., Koutinas, A., Kampioti, A., Eriotou, E., Kopsahelis, N.: Valorization of Zante currant side-streams for the production of phenolic-rich extract and bacterial cellulose: a novel biorefinery concept. J. Chem. Technol. Biotechnol. (2019). https://doi.org/10.1002/jctb.6035
Papadaki, A., Kachrimanidou, V.K., Lappa, I., Andriotis, H., Eriotou, E., Mandala, I., Kopsahelis, N.: Tuning the physical and functional properties of whey protein edible films: effect of pH and inclusion of antioxidants from spent coffee grounds. Sustain. Chem. Pharm. 27, 100700 (2022). https://doi.org/10.1016/j.scp.2022.100700
Kachrimanidou, V., Kopsahelis, N., Chatzifragkou, A., Papanikolaou, S., Yanniotis, S., Kookos, I., Koutinas, A.A.: Utilisation of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock. Waste Biomass Valoriz. 4, 529–537 (2013). https://doi.org/10.1007/s12649-012-9191-x
Liang, C.-H., Ho, K.-J., Huang, L.-Y., Tsai, C.-H., Lin, S.-Y., Mau, J.-L.: Antioxidant properties of fruiting bodies, mycelia, and fermented products of the culinary-medicinal king oyster mushroom, Pleurotus eryngii (higher basidiomycetes), with high ergothioneine content. Int. J. Med. Mushrooms 15, 267–275 (2013). https://doi.org/10.1615/IntJMedMushr.v15.i3.40
Lung, M.Y., Deng, K.W.: Improved production and insulinotropic properties of exopolysaccharide by Phellinus igniarius in submerged cultures. Processes 10, 310 (2022). https://doi.org/10.3390/pr10020310
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951). https://doi.org/10.1016/s0021-9258(19)52451-6
Jo, W.-S., Rew, Y.-H., Choi, S.-G., Seo, G.-S., Sung, J.-M., Uhm, J.-Y.: The culture conditions for the mycelial growth of Phellinus spp. Mycobiology 34, 200–205 (2006). https://doi.org/10.4489/MYCO.2006.34.4.200
Hur, H., Imtiaj, A., Lee, M.W., Lee, T.-S.: Suitable conditions for mycelial growth of Phellinus spp. Mycobiology 36, 152–156 (2008). https://doi.org/10.4489/MYCO.2008.36.3.152
Juers, D.H., Matthews, B.W., Huber, R.E.: LacZ β-galactosidase: Structure and function of an enzyme of historical and molecular biological importance. Protein Sci. 21, 1792–1807 (2012). https://doi.org/10.1002/pro.2165
Lee, J.W., Baek, S.J., Kim, Y.S.: Submerged culture of Phellinus linteus for mass production of polysaccharides. Mycobiology 36, 178 (2008). https://doi.org/10.4489/myco.2008.36.3.178
Wang, Z., Quan, Y., Zhou, F.: Optimization of medium composition for exopolysaccharide production by Phellinus nigricans. Carbohydr. Polym. 105, 200–206 (2014). https://doi.org/10.1016/j.carbpol.2014.01.099
Cho, K., Lee, J., Han, G., Kim, N.K., Bae, H., Hwang, S.: Resource recovery using whey permeate to cultivate Phellinus linteus mycelium: solid-state and submerged liquid fermentation. J. Dairy Sci. 98, 6739–6748 (2015). https://doi.org/10.3168/jds.2015-9631
Vinale, F., Scala, F., Marra, R., Ritieni, A., Cavallo, P., Bolletti, S., Borriello, G., Ruocco, M., Woo, S.L., Lorito, M.: A novel antagonistic strain of Sepedonium chrysospermum A novel antagonistic strain of Sepedonium chrysospermum. J. Nutr. Ecol. Food Res. (2013). https://doi.org/10.1166/jnef.2013.1035
Nagao, K., Yoshida, N., Iwai, K., Sakai, T., Tanaka, M., Miyahara, T.: Production of sepedonin by Sepedonium chrysospermum NT-1 in submerged culture. Environ. Sci. 13, 251–256 (2006)
Antonić, B., Jančíková, S., Dordević, D., Tremlová, B.: Grape pomace valorization: a systematic review and meta-analysis. Foods (Basel, Switzerland) 9, 1627 (2020). https://doi.org/10.3390/foods9111627
Argyropoulos, D., Psallida, C., Sitareniou, P., Flemetakis, E., Diamantopoulou, P.: Biochemical evaluation of Agaricus and Pleurotus strains in batch cultures for production optimization of valuable metabolites. Microorganisms (2022). https://doi.org/10.3390/microorganisms10050964
Diamantopoulou, P., Papanikolaou, S., Kapoti, M., Komaitis, M., Aggelis, G., Philippoussis, A.: Mushroom polysaccharides and lipids synthesized in liquid agitated and static cultures. Part I: screening various mushroom species. Appl. Biochem. Biotechnol. 167, 536–551 (2012). https://doi.org/10.1007/s12010-012-9713-9
Li, X., Jiao, L., Zhang, X., Tian, W., Chen, S., Zhang, L.: Structure of polysaccharides from mycelium and culture medium of Phellinus nigricans using submerged fermentation. Sci. China Ser. C Life Sci. 51, 513–519 (2008). https://doi.org/10.1007/s11427-008-0065-1
Haque, M.A., Kachrimanidou, V., Koutinas, A., Lin, C.S.K.: Valorization of bakery waste for biocolorant and enzyme production by Monascus purpureus. J. Biotechnol. 10(231), 55–64 (2016). https://doi.org/10.1016/j.jbiotec.2016.05.003
Kantifedaki, A., Kachrimanidou, V., Mallouchos, A., Papanikolaou, S., Koutinas, A.A.: Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J. Clean. Prod. 185, 882–890 (2018). https://doi.org/10.1016/j.jclepro.2018.03.032
Acknowledgements
We acknowledge support of this work by the project “Monumental forests of the Ionian Islands as resource areas of biodiversity and high added-value mushrooms: mapping, recording, evaluation, networking, preservation and sustainable exploitation” (MIS 5033680) which is implemented under the Action “Protection of the environment and sustainable development” funded by the Operational Programme “Ionian Islands 2014-2020” and co-financed by Greece and the European Union (European Regional Development Fund). Also, authors would like to thank Mrs. Marina Triantafyllou and Mrs. Elena Magdalinou for their valuable work during mushroom collection.
Funding
Open access funding was provided by HEAL-Link Greece. Funding was provided by the Operational Programme Ionian Islands 2014-2020 (MIS 5033680).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kachrimanidou, V., Papadaki, A., Alexandri, M. et al. Sepedonium sp. and Phellinus sp. Novel Isolates: Growth Pattern and Production of Polysaccharide-Protein Complexes on Conventional and Grape Pomace Substrates. Waste Biomass Valor 14, 3315–3326 (2023). https://doi.org/10.1007/s12649-022-02017-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-02017-z