Abstract
The lichenicolous anamorphic fungus Sclerococcum parmeliae was isolated in pure culture, and ITS, nuLSU and mtSSU sequences were obtained from these isolates. For comparison, sequences from S. sphaerale, the generic type, were obtained directly from freshly collected specimens. Phylogenetic analyses place S. sphaerale with species of Dactylospora and an unidentified lichen-inhabiting isolate in a strongly supported clade that is sister to a lineage comprising members of the Chaetothyriales and Pyrenulales. In contrast, S. parmeliae is inferred as a member of the Herpotrichiellaceae (Chaetothyriales) and belongs to a robustly supported clade that also includes species of Cladophialophora, Capronia semiimmersa, and Phialophora verrucosa. Within the Herpotrichiellaceae, S. parmeliae most closely resembles members of the anamorph genus Cladophialophora. Accordingly, we propose the transfer of S. parmeliae and the morphologically similar species S. cladoniae, S. hawksworthii and S. normandinae to Cladophialophora. A new lichenicolous species, Clad. megalosporae, collected twice on Megalospora in Florida and Papua New Guinea, is also described.
Similar content being viewed by others
Introduction
The genus Sclerococcum Fr. was originally described for the single lichenicolous hyphomycete S. sphaerale (Ach. : Fr.) Fr., a species that forms conspicuous, blackish, compact, stromatic sporodochia on the thallus of Pertusaria gr. corallina, in which dark brown, 2–6-celled conidia are produced in irregular chains (Hawksworth 1975, 1979). Fifteen additional species of Sclerococcum have been described since; all but one member of the genus are lichenicolous, and most species are confined to a single host species or genus (see http://www.lichenicolous.net). In addition to a number of species with blackish, compact sporodochia (keyed out by Etayo and Calatayud 1998), several other species with quite loose, greyish or brown sporodochia and irregularly catenate 0–1-septate conidia that separate easily in squash preparations have been described in Sclerococcum, including S. cladoniae Diederich, S. hawksworthii Etayo & Diederich, S. normandinae Diederich & Etayo and S. parmeliae Etayo & Diederich (Etayo and Diederich 1995, 1996). Sclerococcum griseosporodochium Etayo was described as possibly lichenicolous over saxicolous lichens (Etayo 1995) but it is now regarded as a lichenized, non-lichenicolous species associated with a Trentepohlia photobiont (Smith 2009).
Although morphological variation among species of Scleroccocum suggests that the genus is strongly heterogeneous, molecular data are needed to assess the phylogenetic position of S. sphaerale, the generic type, and other representatives of the genus. The aim of this study was to investigate for the first time the position of S. sphaerale within the Ascomycota using molecular data and to determine if this species is closely related to S. parmeliae, a member of the genus that produces catenate, disarticulating conidia.
Materials and methods
Morphological study
Herbarium specimens are deposited in BR, DBN, LG, M and NY, and in the private collections of P. Diederich and F. Berger. Microscopical examination (including all microscopical measurements) was carried out using thin hand-made sections mounted in water. Conidial measurements of the new species are indicated as (minimum–)\( \overline {\text{X}} - {\sigma_{\text{X}}} - \overline {\text{X}} { + }{\sigma_{\text{X}}} \)(–maximum), followed by the number of measurements (n); the length/breadth ratio of conidia is indicated as l/b and given in the same way.
Isolation of fungal cultures
Conidiomata of S. parmeliae were isolated from lichen host tissues in 70 % ethanol, dried on a glass slide, and crushed in sterile water; conidia were then collected in water and plated onto Maltose Extract agar or Potato Dextrose agar (Difco, Detroit, Michigan, USA) lacking antibiotics, where they were observed to germinate within 7 days. Conidiomata of freshly collected material of Sclerococcum sphaerale were crushed in sterilized water, and conidia transferred to Malt-Yeast Extract agar following Yoshimura et al. (2002). Germination occurred within a few days, but the resulting cultures ceased growing on this medium after a week.
Molecular techniques
Cultures of Sclerococcum parmeliae (CBS 129337) were grown on Sabouraud’s agar with maltose (SMA, Difco, Detroit, Michigan, USA) for 2 weeks. Genomic DNA was extracted from harvested mycelia using the Bio 101 Fast DNA Spin Kit for tissue (Qbiogen, Illkirch, France) according to the manufacturer’s protocol with slight modifications. Approximately 10 ng of extracted DNA were subjected to a standard PCR in a 25 μL reaction volume using either Taq Gold polymerase (Applied Biosystems, Foster City, California, USA) or Bio-X-Act Long Mix (Bioline USA, Inc., Taunton, Massachusetts, USA). We amplified and sequenced approximately 1,700 bp of the nuLSU using primers LR0R, LR3R, LR8R, LR5, LR7 and LR9, and for S. parmeliae (CBS 129337), the ITS using primers ITS5 and ITS4 (available on the Duke Mycology website, http://www.biology.duke.edu/fungi/mycolab/primers.htm). Primers for amplification and sequencing of the mtSSU rDNA were mrSSU1 and mrSSU3R (Zoller et al. 1999). Hyphae of the second culture of S. parmeliae (CBS 132232) were added to a tube containing the PCR reaction mixture and amplified directly, for the nuLSU using LIC15R and LR6 (available on the Duke Mycology website, http://www.biology.duke.edu/fungi/mycolab/primers.htm) and for the mtSSU rDNA using mrSSU1 and mrSSU3R. The method of direct PCR as explained in Lawrey et al. (2007: 780) was performed on the conidiomata of three freshly collected specimens of Sclerococcum sphaerale using the same primers as for S. parmeliae (CBS 132232).
PCR products were visualized in a 1 % agarose gel with ethidium bromide, purified with Ampure magnetic beads (Agencourt Biosciences, Beverly, Massachusetts, USA) following the manufacturer’s instructions, and used in standard sequencing reactions with BigDye Terminator Ready Reaction Mix v3.1 (Applied Biosystems). Reactions were then purified using Sephadex G-50 (Sigma-Aldrich, St. Louis, Missouri, USA), dried in a speedvac, denatured in HiDi Formamide (Applied Biosystems), and sequenced using a 3130xl Genetic Analyzer (Applied Biosystems). Sequences were analyzed using Sequence Analysis v5.4 (Applied Biosystems) and were manually edited using Sequencher v4.7 (Gene Codes Corporation, Ann Arbor, Michigan, USA), and overlapping fragments were assembled in larger consensus sequences.
Taxon selection and phylogenetic analyses
Taxa selected for phylogenetic analyses (Table 1) included members of the Chaetothyriales published by Crous et al. (2007), Gueidan et al. (2008), Ruibal et al. (2005, 2008), and Untereiner and Naveau (1999), as well as those retrieved from GenBank based on searches of the newly generated sequences of Sclerococcum using BLAST (Altschul et al. 1990). Major groups represented in these phylogenies include the Herpotrichiellaceae, a number of the strongly supported lineages basal to this family that were recognized by Badali et al. (2008) and Untereiner et al. (2011), and clades corresponding to the Coryneliales, Eurotiales, Lichinales, Mycocaliciales, Onygenales and Pyrenulales.
Alignments were generated with Clustal X 1.82 (Jeanmougin et al. 1998) and edited using Se-Al 2.0a11 (Rambaut 2002). Multiple base indels were reduced to single characters, and all ambiguously aligned sequences were excluded. The positions of Sclerococcum parmeliae and S. sphaerale within the Ascomycota were inferred based on the analysis of the nuLSU sequences of 51 taxa with Peltula auriculata, P. umbilicata (Lichinales) and Pleopsidium chlorophanum (Lecanoromycetes) as outgroup taxa. We also analyzed ITS sequences of 29 taxa to more accurately position S. parmeliae. Outgroup taxa for this analysis included Capronia pilosella and C. pulcherrima.
Maximum parsimony (MP) analyses were performed with PAUP* 4.0b10 (Swofford 2002). Heuristic searches of datasets were performed employing simple sequence addition and TBR branch swapping with the MULTREES option activated. Gaps were defined as a fifth character in all analyses. Bootstrap support (BS) for internal branches was evaluated from 1,000 full heuristic searches using TBR branch swapping but without the MULTREES option activated. Only groups with a frequency of greater than 50 % were retained in consensus trees.
Results and discussion
Phylogenetic analyses
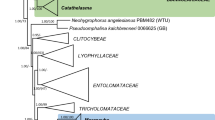
The nuLSU dataset consisted of 1,358 characters of which 332 were parsimony-informative (PI). Analysis of this dataset produced 3 most parsimonious trees (MPTs) 1,195 steps in length (L) with a consistency index (CI) of 0.503 and a retention index and (RI) of 0.765. One of these trees is shown in Fig. 1.
Phylogenetic relationships of Sclerococcum parmeliae and S. sphaerale inferred from nuLSU sequences. This is one of two MPTs inferred from an heuristic search of 1,358 characters for 51 taxa (L = 1195, CI = 0.503, RI = 0.765). Bootstrap values greater than 50 % calculated from 1,000 full heuristic searches are provided above or adjacent to the corresponding nodes. An asterisk (*) indicates branches supported in 100 % of BS replicates. Vertical bars indicate well-supported lineages within the Eurotiomycetes, Lecanoromycetes, and Lichinomycetes (A = Chaetothyriales, B = Pyrenulales, C = Sclerococcum sphaerale - Dactylospora clade, D = Eurotiales / Onygenales, E = Coryneliales, F = Mycocaliciales, G = Lichinales)
In this phylogeny isolates of Sclerococcum parmeliae were inferred as members of the Chaetothyriales within a strongly supported clade (BS 99 %) that included two rock-inhabiting isolates (TRN486, TRN531), Capronia semiimmersa, Cladophialophora carrionii, Clad. boppii, Clad. chaetospira, and Phialophora verrucosa. The close relationship of S. parmeliae to these species is also supported by BLAST searches of the mtSSU sequences of CBS 129337 (JQ342181) and CBS 132232 (JX081675) (data not shown). Sclerococcum sphaerale was positioned with species of Dactylospora and an unidentified lichen-inhabiting isolate in a strongly supported clade (BS 100 %) that was basal to a lineage comprising members of the Chaetothyriales and Pyrenulales. BLAST searches of mtSSU sequences of three collections of S. sphaerale (JX081676, JX081677, JX081678) return low values for coverage query (76 %) and maximum identity (92 %) and do not further resolve the relationship of this species to other Eurotiomycetes.
The ITS datasets considered to more precisely position S. parmeliae within the Herpotrichiellaceae consisted of 543 (136 PI) characters. Comparison of ITS sequences for this dataset produced a single MPT (L = 370, CI = 0.643, RI = 0.815) (Fig. 2). This phylogeny confirmed the close relationship of S. parmeliae to TRN486 and TRN531 but it did not resolve the position of this species within a clade (BS <50 %) that also included Clad. chaetospira, three endolichenic isolates, and three unidentified, uncultured Cladophialophora. However, this clade was positioned within a robustly supported lineage (BS 90 %) that included a group comprising isolates of Clad. carrionii, Clad minourae, C. semiimmersa, and P. verrucosa (BS 100 %) and a clade that contained isolates of Clad. boppii (BS 100 %).
Phylogenetic relationship of Sclerococcum parmeliae inferred from ITS sequence data. This is the single MPT inferred from an heuristic search of 543 characters for 29 taxa (L = 370, CI = 0.643, RI = 0.815). Bootstrap values greater than 50 % calculated from 1,000 full heuristic searches are provided above or adjacent to the corresponding nodes. An asterisk (*) indicates branches supported in 100 % of BS replicates
Discussion
Phylogenetic analysis of nuLSU sequences (Fig. 1) resolves Sclerococcum sphaerale, the generic type of Sclerococcum, as a member of a strongly supported clade basal to the Eurotiomycetes that also includes Dactylospora spp. and an unidentified lichenicolous rock isolate (ALr-1). The latter was obtained from sterile dark colored mycelia on the thallus of Lecanora rupicola (Brunauer et al. 2007), and a habit photograph (Fig. 1 in Brunauer et al. 2007) suggests Sclerococcum montagnei Hafellner, a species confined to L. rupicola (Hafellner 1996) that is morphologically similar to S. sphaerale. Unfortunately, the voucher specimen for ALr-1 could not be located in GZU.
The Dactylospora sequences used in our analysis were found to belong to the Eurotiomycetes by Schoch et al. (2009), a result confirmed by us. Dactylospora is therefore not a member of the Lecanorales as has been indicated recently in Index Fungorum, but is obviously heterogeneous, representing at least two distinct clades, one containing D. lobariella and D. imperfecta, and another more basal clade containing the marine species D. mangrovei and D. haliotrepha (Schoch et al. 2009, suppl. Figure 6c). Resolving the phylogenetic position of Dactylosporaceae in the Eurotiomycetes will therefore require information about which of these two clades contains the generic type, D. floerkei Körb. [= D. parasitica (Flörke ex Sprengel) Zopf].
Sclerococcum parmeliae is as a member of the Herpotrichiellaceae (Chaetothyriales, Eurotiomycetes, Ascomycota), a family that encompasses lignicolous and fungicolous Capronia Sacc. and a large number of taxa assigned to the anamorph genera Cladophialophora Borelli, Exophiala J.W. Carmich., Fonsecaea Negroni, and Phialophora Medlar (Müller et al. 1987; Gueidan et al. 2008; Untereiner et al. 2011). Within this family, S. parmeliae belongs to a lineage that includes TRN486 and TRN531, two unnamed rock-inhabiting isolates inferred previously as members of a well-supported group within the “Capronia clade” based on the analysis of ITS sequences (Ruibal et al. 2008). Although our analysis of an expanded set of ITS sequences (Fig. 2) does not identify the closest relatives of S. parmeliae, it unequivocally positions this species within a strongly supported clade that includes Clad. carrionii (= Clad. ajelloi Borelli), the type species of Cladophialophora.
Sclerococcum parmeliae is not closely related to Capronia peltigerae, another lichenicolous species included in our analysis. Our nuLSU phylogeny is consistent with a previous investigation (Untereiner et al. 2011) that resolved C. peltigerae as a member of a well-supported lineage basal to the Herpotrichiellaceae. Capronia, as circumscribed currently, is polyphyletic but it will not be possible to determine if C. peltigerae represents a novel taxon within the Chaetothyriales until additional lichenicolous representatives of the genus are cultured and included in molecular phylogenetic studies. That being said, it is noteworthy that lichenicolous species of Sclerococcum have repeatedly been observed on lichen thalli that also bear ascomata of lichenicolous Capronia. For example, C. hypotrachynae Etayo & Diederich and S. parmeliae occur on a thallus of Hypotrachyna collected in the Isle of Pico, Azores (Diederich 17092), and Etayo (2002) reported both C. hypotrachynae and S. parmeliae on the same lichen thalli from five localities in Colombia. Capronia normandinae R. Sant. & D. Hawksw. and S. normandinae were also found on the same thallus of Normandina pulchella collected in the French Pyrénées-Atlantiques (gorges de Kakouetta) (Diederich 9560). Although it is interesting to speculate that S. normandinae and S. parmeliae are the anamorphs of species of Capronia, these connections have yet to be established based on morphological observations and molecular data.
Our phylogenetic analyses demonstrate that Sclerococcum parmeliae is not congeneric with the type of Sclerococcum and should be transferred to another genus. Sclerococcum parmeliae and S. sphaerale can also be differentiated morphologically. Conidiomata of S. sphaerale are blackish, weakly stromatic sporodochia that produce irregular, nearly biseriate chains of subglobose to ellipsoidal, multicellular conidia (Fig. 3). Hawksworth and Jones (1981) obtained S. sphaerale in axenic culture and reported the in vitro production of conidia to be comparable to what is observed on the host. In contrast, S. parmeliae forms loose, non-stromatic sporodochia that give rise to masses of irregularly catenate, ellipsoidal, 1-septate conidia that adhere in short chains (Fig. 5). Among the related anamorphic Herpotrichiellaceae, S. parmeliae most closely resembles Cladophialophora, a genus characterized by the production of poorly differentiated conidiophores that give rise to branched chains of dry, strongly coherent, moderately melanized, 1(–3) celled, ellipsoidal to fusiform conidia (Badali et al. 2008, 2009; de Hoog et al. 2007). Most members of the genus cause opportunistic infections of vertebrates but a number of phytopathogenic and saprobic species have been described (Crous et al. 2007; de Hoog et al. 2007; Badali et al. 2009). Sclerococcum parmeliae can be distinguished from the type species Clad. carrionii (with aseptate conidia) and from Clad. chaetospira (with 1(–3)-septate conidia), the two members of the genus to which it is most closely related (Fig. 2), by its 1-septate, verrucose conidia that frequently remain attached to each other laterally (Fig. 5d–f). The resulting chains of conidia are typically strongly curved and flexuous rather than straight. Several other species of Sclerococcum, viz. S. cladoniae, S. hawksworthii and S. normandinae, resemble S. parmeliae in the morphology of their conidiomata and mode of conidiogenesis, but they differ in forming aseptate, often smaller and smoother conidia. Although molecular data are not yet available for these species, they are sufficiently similar to S. parmeliae to warrant their combination in Cladophialophora.
Taxonomy
Sclerococcum Fr. : Fr.
Syst. mycol. 1: xl (1821). Type: S. sphaerale (Ach. : Fr.) Fr.
Sclerococcum sphaerale (Ach. : Fr.) Fr. (Fig. 3)
Ach., Syn. Lich.: 2 (1814); Fries, Syst. mycol. 3: 257 (1832).
MycoBank 206581
For typification, description and illustrations, see Hawksworth (1975).
Selected specimens examined: Belgium: Ardennes, SW of Nadrin, Le Hérou, 2012, Ertz 17425 (BR). Luxembourg: Berdorf, Binzeltschlëff, on Pertusaria corallina, 1984, Diederich 5745 (hb Diederich); ibid., 2012, Diederich 17279 (BR, hb Diederich); Berdorf, Wanterbaach, 2012, Diederich 17283 (BR, hb Diederich).
Cladophialophora Borelli
Proc. 5 th Int. Conf. on the Mycoses, PAHO Publ. 396: 335 (1980). Type: C. ajelloi Borelli [= C. carrionii (Trejos) de Hoog et al.].
For illustrations, notes and references, see Seifert et al. (2011). As discussed in the previous section, we are proposing the transfer of Sclerococcum parmeliae and three morphologically similar species of Sclerococcum to Cladophialophora. A fifth species, collected twice on Megalospora, will be described as new. The genus Cladophialophora should therefore be emended to include also species with loose sporodochia. We contemplated transferring these species to Capronia Sacc. (1883), a generic name that precedes Cladophialophora Borelli (1980). However, until the phylogenetic position of the generic type, Capronia sexdecimspora (Cooke) Sacc., is determined, combining these species of Sclerococcum in Capronia is considered as premature.
Cladophialophora cladoniae (Diederich) Diederich comb. nov.
MycoBank MB800397
Basionym: Sclerococcum cladoniae Diederich, Bull. Soc. Natur. Luxemb. 111: 57 (2010). Type: Luxembourg, W of Kayl, Monument des mineurs, alt. 370 m, over mosses in a disused quarry, on Cladonia pocillum and C. subulata, 1 Sep. 2009, P. Diederich 16826 (BR–holotype!; HAL, hb Diederich–isotypes).
For a description and illustrations, see Diederich (2010).
Cladophialophora hawksworthii (Etayo & Diederich) Diederich comb. nov. (Fig. 4h–k)
a–g. Cladophialophora megalosporae (a,d: Aptroot 32016; b–c, e–g: holotype). a. Sporodochia developing on the thallus of Megalospora sp. b. Sporodochia developing on the thallus of M. pachycheila. c. Section through sporodochium. d–e. Irregularly catenate conidia arising from conidiophores (below the centre of d). f. Chains of conidia. g. Conidia in surface view, showing the smooth wall. h–k. Cladophialophora hawksworthii (Diederich 12221). h. Minuscule sporodochia on the thallus of Megalospora tuberculosa (note the acicular crystals of zeorin appearing in herbarium specimens). i–k. Irregularly catenate conidia, some collapsed in old herbarium specimen, in k surface view, showing a smooth to slightly ornamented wall. l–o. Cladophialophora normandinae (Diederich 9567). l. Sporodochium in lateral view on thallus of Normandina pulchella. m–o. Irregularly catenate conidia, in o surface view, showing a strongly verrucose wall. Scale bars: a, l = 500 μm; b = 200 μm; h = 100 μm; c = 20 μm; d–e, i, m = 10 μm; f–g, j–k, n–o = 2 μm
MycoBank MB800398
Basionym: Sclerococcum hawksworthii Etayo & Diederich, in Daniels et al., Flechten Follmann: 217 (1995). Type: France, Pyrénées-Atlantiques, Ibarre, near St-Jean-Pied-de Port, on Quercus robur, on Megalospora tuberculosa, 25 June 1992, J. Etayo 2647 & C. Printzen (MA-Lichen–holotype; hb Etayo–isotype).
For a description and illustrations, see Etayo and Diederich (1995).
Specimen examined: France: Pyrénées-Atlantiques: au sud de Tardets-Sorholus, Ste-Engrâce, vers Pierre-St-Martin, à 3 km après la dernière maison, on Fagus, on Megalospora tuberculosa, 1991, Diederich 12221 & Etayo (hb Diederich).
Cladophialophora megalosporae Diederich sp. nov. (Fig. 4a–g)
MycoBank MB800399
Type: U.S.A., Florida, Liberty Co., hardwoods on W side of Ochlockonee River at Forest Hwy. 13 bridge, 21.5 mi E of Wilma, on Megalospora pachycheila, 28 Dec. 1990, R. C. Harris 26148 (NY–holotype; hb Diederich–isotype).
Colonies forming discrete, minuscule conidiomata on the host thallus; mycelium immersed, brownish, macroscopically not visible. Conidiophores semi-macronematous, pale to medium brown or reddish brown, aggregated into irregularly rounded, convex, medium to dark brown or greyish brown, simple, partly immersed to superficial sporodochia of 20–100(–200) μm diam. Conidiogenous cells monoblastic or polyblastic, integrated, terminal, brown, not clearly defined, and the terminal cells acting in turn as conidiogenous cells. Conidia irregularly adhering in short, branched, acropetal chains, dry, subspherical, pale to medium brown, color not changing in 5 % KOH (or becoming slightly olivaceous), aseptate, smooth-walled, thin-walled, (2.2–)2.4–2.9(–3.3) × (1.9–)2.1–2.6(–3.0) μm, ratio l/b (1.0–)1.0–1.2(–1.4) (n = 40).
Microscopically, this species is very similar to Cladophialophora cladoniae. It is distinguished by the much larger conidiomata, those of C. cladoniae being 7–20(–30) μm diam. As both known specimens of this species occur on hosts belonging to the genus Megalospora, we initially expected that they might belong to C. hawksworthii, a species known only from M. tuberculosa. Macroscopically, conidiomata of both taxa are similar in size, but those of C. megalosporae are more blackish (Fig. 5a–b), while those of C. hawksworthii appear more greyish brown with a slight reddish tinge (Fig. 5h). Microscopically, conidia of C. hawksworthii are distinctly larger (2.5–4 μm diam.) than those of the new species. Furthermore, conidia of C. hawksworthii are more greyish brown, often with a darker wall in optical section (Fig. 5i–k), whereas those of the new species are reddish brown, with a less visible wall (Fig. 5d–g).
Cladophialophora parmeliae. a–b. Sporodochia developing on the thallus of Hypotrachyna imbricatula. c. Section through sporodochium. d. Irregularly catenate, 1-septate conidia. e. Chains of conidia, showing the lateral attachment. f. Conidia in surface view, showing the verrucose ornamentation. g. 10–week-old culture. h. Section through culture. (a, c–d: Diederich 17055; b: culture JL–477 from Diederich 17055). Scale bars: g = 1 mm; a–b = 200 μm; c, h = 20 μm; d = 10 μm; e–f = 5 μm
Additional specimen examined: Papua New Guinea: Madang Province: Huon Peninsula, Finisterre range, Yupna valley, Teptep village, 5°57′ S, 146°33′ E, alt. 2,500 m, disturbed mountain forest, mossy mountain forest and gardens near village, on Megalospora, 1992, Aptroot 32016 (M–0045424, hb Diederich).
Cladophialophora normandinae (Diederich & Etayo) Diederich comb. nov. (Fig. 4l–o)
MycoBank MB800400
Basionym: Sclerococcum normandinae Diederich & Etayo, in Etayo & Diederich, in Daniels et al., Flechten Follmann: 218 (1995). Type: France, Pyrénées-Atlantiques, au sud de Tardets-Sorholus, Ste-Engrâce, vers Pierre-St-Martin, à 3 km après la dernière maison, on Fagus, on Normandina pulchella, 26 July 1990, P. Diederich 9388 (LG–holotype; hb Diederich–isotype!).
For a description and illustrations, see Etayo and Diederich (1995). Specimens from S Chile reported by Etayo and Sancho (2008) as Sclerococcum cf. normandinae, mainly growing on Nephroma, differ by smaller conidiomata, 50–150 μm diam., and by the presence of 20 % of 1-septate conidia; they might represent a distinct, yet undescribed species.
Additional specimens examined: France: Pyrénées-Atlantiques: S of Tardets-Sorholus, Ste-Engrâce, gorges de Kakouetta, on Crataegus, on Normandina pulchella, 1991, Diederich 9560, 9567 & Etayo (hb Diederich).—United Kingdom, Scotland: Mid Ebudes, VC 103: Isle of Mull, overlooking Sound of Ulva, hazel wood SE of Oskamull, NM463398, on N. pulchella, 1999, H. Fox 247 (DBN).
Cladophialophora parmeliae (Etayo & Diederich) Diederich & Untereiner comb. nov. (Fig. 5)
MycoBank MB800401
Basionym: Sclerococcum parmeliae Etayo & Diederich, Mycotaxon 60: 425 (1996). Type: Spain, Navarra, valle de Basaburúa Mayor, between Aizároz and Arrarás, track to Bergañe, alt. 550 m, on Fagus, on Parmelia saxatilis, 20 Sep. 1994, J. Etayo 12688 (MA-Lichen–holotype; hb Etayo–isotype, non vid.).
For a description and illustrations, see Etayo and Diederich (1996).
Specimens examined: Austria: Oberösterreich: Donautal, Schlögener Schlinge, Steiner Fels, 2185/66, MTB 7549, alt. 380 m, on Flavoparmelia caperata, 1994, Berger 8149 (hb Berger).—France: Pyrénées-Atlantiques: col de Lizuniaga, c. 5 km from Bera de Bidasoa, on F. caperata, 1994, Etayo 12674 (hb Diederich).—Spain, Azores: Pico: S of Sao Roque do Pico, forest remnants on the shore of Lagoa Capitao, 38°29′9″ N, 28°18′58″ W, alt. 780 m, on Juniperus brevifolia, on Hypotrachyna imbricatula, 2010, Diederich 17055 (hb Diederich) (culture JL-477, CBS 129337); ibid., on Hypotrachyna, 2011, Ertz 16591 (BR) (culture CBS 132232); N of Lajes do Pico, near Lagoa do Paúl, 38°25′40″ N, 28°13′58″ W, alt. 790 m, on J. brevifolia, on H. imbricatula, 2010, Diederich 17028 (hb Diederich); between Lajes do Pico and Sao Roque do Pico, Bosque da Junqueira, 1 km S of crossing with road going to the east, 38°27′56″ N, 28°17′57″ W, on J. brevifolia in laurisilva, on Hypotrachyna, 2010, Diederich 17092 (hb Diederich).—United Kingdom, Scotland: Isle of Skye: Forest SW of Tokavaig, close to the sea, alt. 5 m, on Lobaria pulmonaria, 2003, Diederich 15659 (hb Diederich).
Key to the lichenicolous species of Cladophialophora
-
1 Conidia mainly 1-septate, ellipsoidal, slightly to distinctly verrucose; conidiomata 50–120 μm diam.; on parmelioid lichens, Lobaria, Normandina, Pannaria and Sticta........................................................................... C. parmeliae
-
1 Conidia aseptate, subspherical to short-ellipsoidal................2
-
2 Conidia 4–6 × 3.5–4 μm, verrucose; conidiomata 150–300 μm diam.; on Normandina pulchella................... C. normandinae
-
2 Conidia smaller, smooth or indistinctly ornamented; conidiomata smaller .................................................................3
-
3 Conidia 2.5–4 μm diam., greyish brown; conidiomata 30–100(–130) μm diam.; on Megalospora tuberculosa...................................................................... C. hawksworthii
-
3 Conidia less than 3 μm diam., reddish brown................4
-
4 Conidiomata 7–20(–30) μm diam.; conidia 2.2–3 μm; on Cladonia......................................................... C. cladoniae
-
4 Conidiomata 20–100(–200) μm diam.; conidia 2.5–3.0 × 2.0–2.5 μm; on Megalospora................. C. megalosporae
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Badali H, Gueidan C, Najafzadeh MJ, Bonifaz A, Gerrits van den Ende AHG, de Hoog GS (2008) Biodiversity of the genus Cladophialophora. Stud Mycol 61:175–191
Badali H, Carvalho VO, Vicente V, Attili-Angelis D, Kwiatkowski IB, Gerrits van den Ende AHG, de Hoog GS (2009) Cladophialophora saturnica sp. nov., a new opportunistic species of Chaetothyriales revealed using molecular methods. Med Mycol 47:55–66
Brunauer G, Blaha J, Hager A, Türk R, Stocker-Wörgötter E, Grube M (2007) An isolated lichenicolous fungus forms lichenoid structures when co-cultured with various coccoid algae. Symbiosis 44:127–136
Crous PW, Schubert K, Braun U, de Hoog GS, Hocking AD, Shin HD, Groenewald JZ (2007) Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Stud Mycol 58:185–217
de Hoog GS, Nishikaku AS, Fernandez-Zeppenfeldt G, Padín-González C, Burger E, Badali H, Richard-Yegres N, Gerrits van den Ende AHG (2007) Molecular analysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Stud Mycol 58:219–234
Diederich P (2010) Sclerococcum cladoniae, a new lichenicolous hyphomycete on Cladonia from Luxembourg. Bull Soc Nat Luxemb 111:57–59
Etayo J (1995) Two new species of lichenicolous fungi from the Pyrenees. Nova Hedwigia 61:189–197
Etayo J (2002) Aportación al conocimiento de los hongos liquenícolas de Colombia. Bibl Lichenol 84:1–154
Etayo J, Calatayud V (1998) Sclerococcum (Deuteromycotina) with black sporodochia in Spain. Ann Naturhist Mus Wien 100B:677–681
Etayo J, Diederich P (1995) Lichenicolous fungi from the western Pyrenees, France and Spain. I. New species of deuteromycetes. In: Daniëls FJA, Schulz M, Peine J (eds) Flechten Follmann. Contributions to lichenology in honour of Gerhard Follmann. Geobotanical and Phytotaxonomical Study Group, Botanical Institute, University of Cologne, Cologne, pp 205–221
Etayo J, Diederich P (1996) Lichenicolous fungi from the western Pyrenees, France and Spain. II. More deuteromycetes. Mycotaxon 60:415–428
Etayo J, Sancho LG (2008) Hongos liquenícolas del Sur de Sudamérica, especialmente de Isla Navarino (Chile). Bibl Lichen 98:1–302
Gueidan C, Ruibal Villaseñor C, de Hoog GS, Gorbushina AA, Untereiner WA, Lutzoni F (2008) A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud Mycol 61:111–119
Hafellner J (1996) Bemerkenswerte Funde von Flechten und lichenicolen Pilzen auf makaronesischen Inseln V. Über einige Neufunde und zwei neue Arten. Herzogia 12:133–145
Hawksworth DL (1975) A revision of lichenicolous fungi accepted by Keissler in Coniothecium. Trans Br Mycol Soc 65:219–238
Hawksworth DL (1979) The lichenicolous hyphomycetes. Bull Br Mus Nat Hist Bot Ser 6:183–300
Hawksworth DL, Jones D (1981) Sclerococcum sphaerale obtained in pure culture. Trans Br Mycol Soc 77:485–489
Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23:403–405
Lawrey JD, Binder M, Diederich P, Molina MC, Sikaroodi M, Ertz D (2007) Phylogenetic diversity of lichen-associated homobasidiomycetes. Mol Phylogenet Evol 44:778–789
Müller E, Petrini O, Fisher PJ, Samuels GJ, Rossman AY (1987) Taxonomy and anamorphs of the Herpotrichiellaceae with notes on generic synonymy. Trans Br Mycol Soc 88:63–74
Rambaut A (2002) Se-Al sequence alignment editor. Version 2.0a11. Available at http://tree.bio.ed.ac/software/seal
Ruibal C, Platas G, Bills GF (2005) Isolation and characterization of melanized fungi from limestone formations in Mallorca. Mycol Prog 4:23–38
Ruibal C, Platas G, Bills GF (2008) High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain. Persoonia 21:93–110
Schoch C, Sung GH, López-Giráldez F, Townsend JP, Miadlikowska J, Hofstetter V, Robbertse B, Matheny B, Kauff F, Wang Z, Gueidan C, Andrie RM, Trippe K, Ciufetti LM, Wynns A, Fraker E, Hodkinson BP, Bonito G, Groenewald JZ, Arzanlou M, De Hoog GS, Crous PW, Hewitt D, Pfister D, Peterson K, Gryzenhout M, Wingfield MJ, Aptroot A, Suh SO, Blackwell M, Hillis DM, Griffith GW, Castlebury LA, Rossman A, Lumbsch HT, Lücking R, Büdel B, Rauhut A, Diederich P, Ertz D, Geiser DM, Hosaka K, Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Mostert L, O’Donnell K, Sipman H, Rogers JD, Shoemaker R, Sugiyama J, Summerbell RC, Untereiner W, Johnston PR, Stenroos S, Zuccaro A, Dyer PS, Crittenden PD, Cole MS, Hansen K, Trappe JM, Yahr R, Lutzoni F, Spatafora JW (2009) The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst Biol 58:224–239
Seifert K, Morgan-Jones G, Gams W, Kendrick B (2011) The genera of hyphomycetes. CBS–KNAW Fungal Biodiversity Centre, Utrecht
Smith CW (2009) Sclerococcum Fr. (1825). In: Smith CW et al (eds) The Lichens of Great Britain and Ireland. The British Lichen Society, London, p 837
Swofford DL (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland
Untereiner WA, Naveau FA (1999) Molecular systematics of the Herpotrichiellaceae with an assessment of the phylogenetic positions of Exophiala dermatitidis and Phialophora americana. Mycologia 91:67–83
Untereiner WA, Gueidan C, Orr MJ, Diederich P (2011) The phylogenetic position of the lichenicolous ascomycete Capronia peltigerae. Fungal Divers 49:225–233
Yoshimura I, Yamamoto Y, Nakano T, Finnie J (2002) Isolation and culture of lichen photobionts and mycobionts. In: Kranner I, Beckett RP, Varma AK (eds) Protocols in Lichenology: culturing, biochemistry, physiology and use in biomonitoring. Springer, Berlin, pp 3–33
Zoller S, Scheidegger C, Sperisen C (1999) PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 31:511–516
Acknowledgments
We thank the curators of herbaria and the owners of private collections for the loan of specimens in their care, and the curator of GZU for searching for the voucher specimen of culture ALr-1. This study was supported by NSF grant DEB 0841405 to JDL and a Discovery Grant to WAU from the Natural Science and Engineering Research Council (NSERC) of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diederich, P., Ertz, D., Lawrey, J.D. et al. Molecular data place the hyphomycetous lichenicolous genus Sclerococcum close to Dactylospora (Eurotiomycetes) and S. parmeliae in Cladophialophora (Chaetothyriales). Fungal Diversity 58, 61–72 (2013). https://doi.org/10.1007/s13225-012-0179-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-012-0179-4