Abstract
Purpose of Review
Gremmeniella abietina is a destructive forest pathogen responsible for Scleroderris canker, shoot dieback, defoliation, and tree death in forests and tree nurseries. This review is aimed at providing a complete description of the fungus, its distribution, the conditions for its spread, and the impact of climate change and at summarising the relevant forest management methods. Due to the worldwide importance of the pathogen, a retrospective review is required to summarise the lessons learned in relation to the disease, considering application to future outbreaks.
Recent Findings
We revise available management methods, considering examples of control strategies, with special focus on the silvicultural approaches, and we also revise the recovery of the affected stands and the associated trade-offs. Forest disturbances such as pests and disease outbreaks are expected to be exacerbated by climate change, although the exact impact on all host-pathogen interactions remains unclear. In regions with a high risk of G. abietina epidemics, climate change is expected to affect the pathogen differently.
Summary
Gremmeniella abietina is a widely distributed forest pathogen in Europe and is also present in North America. Based on the conclusions reached in this review, forest stands may recover from pathogen outbreaks within 10 years, with considerable loss of growth and the risk of attack from secondary factors. Provenance selection is vital for preventing outbreaks. Climate change is expected to have different effects: in some areas, it is likely to increase the conditions conducive to the development of the fungus, while in others, it is likely to limit the spread because of high temperatures and low humidity. Preventing future outbreaks of this pathogen requires the use of mitigating strategies, together with forest monitoring, forecasting, and planning.
Similar content being viewed by others
Introduction and Distribution of the Pathogen
Gremmeniella abietina (Lagerb.) M. Morelet is a haploid ascomycete and a known forest pathogen that produces shoot dieback and cankers on the branches and trunks of conifer trees. It is responsible for the destruction of many seedlings, sample, and adult trees in tree nurseries, conifer plantations, and natural forests throughout the northern hemisphere (Fig. 1), including Southern, Central, and Northern Europe, North America, and Japan [1,2,3,4,5,6,7,8,9]. One of the most recent cases of an epidemic of G. abietina was recorded in Sweden, where more than 400,000 ha of pine forest (including some where sanitation cutting had been conducted) were affected by the disease between 2001 and 2003 [10•]. To date, almost 50 species of conifers belonging to seven different genera have been recognised as possible hosts of Gremmeniella spp., although these pathogens mainly attack members of the genera Abies, Larix, Picea, Juniperus, and Pinus [11]. The fungus causes shoot death, cankers, and bark damage, eventually leading to death of the affected trees, although the extent of the damage may depend on host susceptibility and environmental conditions [12]. In tree nurseries, G. abietina has been reported to cause serious damage to seedlings that can negatively affect annual production of the plants [13], while asymptomatically infected plants endanger future stands, as they act as carriers of the disease [14]. As in the case of Pinus cembra in the Swiss Prealps [15], tree seedlings may also be highly susceptible to this disease in afforestation in sites where the fungus occurs naturally.
Map showing the global distribution of Gremmeniella abietina (based on the EPPO Global Database, accessed February 2023, www.eppo.int) and scheme of Gremmeniella taxonomy; species, races, and biotypes and its common host species (created with BioRender.com)
In general, G. abietina is most aggressive in species growing towards the limit of their range [11], with the exception of plantations where seedlings were infected in tree nurseries. In Europe, this pathogen was included in Annex IIB of Council Directive 2000/29/EC [16] regarding protective measures against the introduction into the community of organisms harmful to plants and against their spread within the community, until this was replaced by Regulation (EU) 2016/2031, and the pathogen was no longer considered a quarantine pest [17].
Nomenclature
The fungus’s nomenclature has changed several times in the last century. All of the synonyms are listed in the U.S. National Fungus Collections Nomenclature Database, as shown below [18]:
Gremmeniella abietina (Lagerb.) M. Morelet 1969 (Ascomycetes, Helotiales)
≡ Crumenula abietina Lagerb. 1913
≡ Ascocalyx abietina (Lagerb.) Schläpfer 1969? [1968] Note: Invalid via ICBN Art. 33.4 Note 1.
≡ Lagerbergia abietina (Lagerb.) J. Reid ex Dennis 1971 Note: Not verified with original lit.
≡ Scleroderris abietina (Lagerb.) Gremmen 1953 Note: Not Ellis & Everh. 1897. Would be a nomen illeg. However, it is invalid via ICBN Art. 33.4 Note 1.
= Brunchorstia destruens Erikss. 1891
= Scleroderris lagerbergii Gremmen 1955 Note: Nom. nov. since the epithet ‘abietina’ is occupied in Scleroderris. However, invalid via ICBN Art. 33.4.
= Crumenula pinea Ferd. & C.A. Jørg. 1939 Note: As (P. Karst.) Ferd. & C.A. Jørg. comb. nov., but it is a new teleomorph name according to ICBN Art. 59.
= Septoria pinea P. Karst. 1884
≡ Brunchorstia pinea (P. Karst.) Höhn. 1915
≡ Excipulina pinea (P. Karst.) Höhn. 1903
The nomenclature of the imperfect or anamorphic phase was first described by Karsten in Finland in 1884 [19], who called it Septoria (Rhabdospora) pinea Karst. However, in 1903, Höhnel [20] moved the taxon to the genus Excipulina, and it then became E. pinea (Karst.) Höhn. In 1888, in Norway, Brunchorst [21] also described the fungus without being aware of the description already made by Karsten, which Eriksson [22] attributed to Brunchorstia destruens Erikss. As a result of the nomenclature rules at that time, the name was then changed to Brunchorstia pinea (P. Karst.) Höhn [23]. The Swedish forest pathologist Lagerberg (1913) linked Brunchorstia pinea (then B. destruens) to an ascomycete found on damaged branches and that he originally identified as Crumenula pinicola (Rebent.) P. Karst. However, although the fungus was very similar to C. pinicola, it was a new species, and he therefore renamed it Crumenula abietina Lagerb [24]. Nevertheless, on the basis of various taxonomic studies, the name was changed to Scleroderris abietina (Lagerb.) Gremmen [25], and 2 years later, Gremmen (1955) suggested the name Scleroderris lagerbergii Gremmen for the same species [26]. However, Schlaepfer–Bernhard (1968) [27] moved this species to the genus Ascocalyx, and the name became Ascocalyx abietina (Lagerb.) Schlaepfer. One year later, Morelet (1969) defined the new genus Gremmeniella on realizing that this fungus was different from both Godronia and Ascocalyx. This genus was then considered monospecific, and the type species was Gremmeniella abietina (Lagerb.) Morelet. [28]. The use of separate names for anamorphs was subsequently discontinued [29], as the dual nomenclature system made accurate diagnoses and legislation difficult for forest pathologists [30]. Therefore, the name Gremmeniella abietina has been specifically recommended to be used as a contribution to the ‘one fungus-one name’ process [31] for use in cases where two or more generic names are synonyms or taxonomically congruent.
Taxonomy of the Genus
The great variability displayed by G. abietina was already indicated by Ettlinger in 1945 [32], who described differences in the number of septa of the conidia of isolates from different localities. The taxonomic classification of the genus Gremmeniella has not yet been completed. It is currently based on the host species, species geography, epidemiology, physiology, and morphology, as well as the findings of serological, genetical, and biochemical studies [33, 34]. Thus far, Gremmeniella has been further divided into five species according to the host tree (Fig. 1): (1) G. abietina (Lagerberg) Morelet, affecting pines, spruces, and firs; (2) G. laricina (Ettinger), found on Larix spp.; (3) G. juniperina L. Holm & Holm on Juniperus spp. [33]; (4) G. balsamea Laflamme & Smerlis sp. nov. (old G. abietina var. balsamea), found on balsam fir [Abies balsamea (L.) Mill] [35]; and (5) Gremmeniella pinicola (Kondo and Kobayashi) Morelet comb. nov., a new combination that was included in the genus Ascocalyx and collected from Pinus taeda [36, 37].
Within the species of G. abietina, one variety (G. abietina var. abietina) is currently recognised (there were previously two varieties: G. abietina var. abietina and G. abietina. var. balsamea) on the basis of morphological characteristics and different molecular markers [38,39,40]. Regarding G. abietina var. abietina, three races have been described on the basis of symptomatic and morphological characteristics [38, 41], biochemical and serological properties [42, 43], as well as genetic differences [34, 39, 44,45,46]: (1) the Asian, (2) North American (NA), and (3) European (EU) races (Fig. 1). Nevertheless, the races of G. abietina var. abietina are probably not only races but form at least two different species.
The Asian race has only been isolated from Abies sachalinensis (F. Schmidt) Mast. in Japan [2]. The NA race is widespread throughout the northern part of the American continent, and it is believed to be specific to natural stands of Pinus contorta Doug ex Loud, P. resinosa Aiton, and P. banksiana Lamb. [47]. The NA race infects young pines and lower branches under snow during winter, resulting in cankers on the trunk, drying branches, and death of the tree, with sexual and asexual stages produced [41, 48, 49]. The NA race appears to be absent in Europe [33, 40], while the EU race is native to Europe, and has been introduced in North America, where it was first detected in the state of New York in 1977 [50] and has since caused serious losses [7]. The EU race attacks different species of the genus Pinus and Larix and has a much broader distribution than that of the other races. In the EU race, three biotypes have been determined on the basis of the length of spores, number of septa, disease symptoms, and molecular markers (Fig. 2): biotype A (also known as LTT, large tree type), biotype B or the Northern biotype (STT, small tree type), and the alpine biotype [6, 40, 46, 51, 52]. The hybridization barrier between A- and B-types has been demonstrated experimentally: artificial pairings between these biotypes revealed low rates of successful germination and growth [47] indicating that these biotypes should be separated into two different species [53, 54]. Population genetic studies have consistently found highly similar alleles in the B and Alpine biotypes [55••].
Life cycle of Gremmeniella abietina: infection of twigs, entrance through stomata, invasion during the winter, formation of pycnidia and dried twigs, and spore dispersion by raindrops and snow by (A) apothecia that creates ascospores (sexual spores) or (B) pycnidia that release conidia (asexual) (created with BioRender.com)
The A biotype is the most virulent type [56], and is the most widespread in Europe, occurring between southern Europe (Spain, Italy, Serbia, Montenegro, and Turkey) [57] and northern Europe, infecting (among other species) P. resinosa, P. sylvestris L., P. contorta, P. nigra J.F. Arnold, P. halepensis Mill, and Picea abies (L.) H. Karst. This biotype scarcely produces apothecia in the field [58]. It also occurs in North America where it is designated as the EU race and was assumed to be introduced via infected seedlings in the period 1950–1960 [11, 34]. This type mainly presents three septa in the conidia, although this character is variable [59]. The B biotype is restricted to relatively high altitudes and latitudes in northern Europe. It especially attacks small trees of Picea abies, Pinus sylvestris, and P. contorta. Furthermore, this biotype produces apothecia in the field, and the number of septa in the conidia ranges from 3 to 7 [52]. The Alpine biotype (previously known as Brunchorstia pinea var. cembrae) has been found exclusively at high altitudes (~ 1800–2000 m) in southern Europe, particularly in the Alps. It infects Pinus cembra L., Pinus mugo Turra, Larix decidua Miller, and also, P. sylvestris [40]. Both the B and Alpine biotypes thrive in very harsh environmental conditions [60], and as remarked before, they are genetically very similar. In addition, the pycnidia and apothecia of both biotypes only appear in buds of seedlings or lower branches of adult trees covered by snow during the winter [52]. Likewise, the Spanish population of G. abietina is genetically unique, clearly differing from the two B and the Alpine types, but related to biotype A in Europe [55, 61], suggesting the putative existence of a fourth biotype within the EU race, or, as proposed before, a specialised population of the biotype A as a result of a genetic bottleneck [11, 57]. Because the grouping of Gremmeniella have not always been done under the International Code of Nomenclature, it is urgent to clarify the status of these groups of Gremmeniella under taxonomical rules.
Life Cycle, Symptoms, and Reproductive Structures
The life cycle of Gremmeniella abietina lasts 2 years [62]. Infection initially takes place during spring when tree buds are developing (Fig. 2). However, the fungus does not develop aggressively until the following winter when the tree is in a dormant state [63]. The first symptoms appear at this time, with loss of the oldest needles, exudation of resin in the buds, and necrosis of tissues caused by advancement of the pathogen [64, 65]. In the following spring, some buds do not sprout, or are deformed, and the needles dry up, resulting in defoliation of the crown and the typical terminal twig distortion (Figs. 3 and 4) [65]. Death of lower branches and the yellowing of needle bases on infected branches have also reportedly been caused by G. abietina [66]. Furthermore, the presence of cankers in twigs and/or in trunks is also frequent (Figs. 3 and 4) especially in the NA race (on the lower portion of the crown) and A biotype, although for instance the Spanish biotype produces them only rarely or not at all [5, 8, 47, 67].
Gremmeniella abietina symptoms: a canker on Pinus resinosa caused by European race (A-type); b shoot blight developing in the snow in Pinus banksiana natural regeneration, North American race; c shoot blight on P. resinosa, European race (A-type); d shoot blight on pine seedlings, North American race; e shoot blight in spring on red pine (P. resinosa), North American race; f mortality on pine trees, European race (A-type) in New York state; g shoot blight in summer on red pine (P. resinosa), European race (A-type). (Pictures Canadian Forest Service-G. Laflamme)
Gremmeniella abietina European race symptoms: a dry needles, crown defoliation (A-type); b terminal twig distortion (A-type); c apothecia (B-type); d asexual fruiting bodies (A-type); e conidia (A-type); f mycelium in pure culture (A-type) (pictures a, b, d–f, C. Romeralo; c, by Canadian Forest Service)
During the first year of infection, in some areas, the damage and symptoms produced by G. abietina are similar to the winter damage caused by adverse weather conditions, and it is therefore very difficult to distinguish one from the other until fruiting bodies appear [68]. However, this does not occur in northern Europe, where symptoms caused by G. abietina can easily be differentiated from winter damage, although they may be confused with damage caused by other fungal pathogens such as Lophodermium seditiosum Minter, Staley & Millar or Sphaeropsis sapinea (Fr.) Dyko & B. Sutton [69]. The asexual fruiting bodies (the pycnidia) are formed in late spring or early summer of the first year, although pycnidia have been reported to appear 2 years after infection [70], and can appear alone or in groups on trunks, branches or insertion of the needles [71]. Asexual fruiting bodies are dark brown or black, stromatic, multilocular, and up to 1 mm in diameter. The conidiophores are hyaline, simple or branched and septate, cylindrical, and measure 10–15 × 2–3 microns. Conidia are hyaline, cylindrical, sometimes curved, mostly with three septa and measure 25–40 × 3–3.5 microns [68]. The survival period of the conidia in the EU race of G. abietina has been reported to be more than 18 months on Pinus sylvestris slash in Sweden [72•] and 2 years on Pinus resinosa slash in Canada [73•].
The sexual fruiting bodies (the apothecia) emerge in the following spring, 2 years after the infection [74], and they mature in the middle of the summer of that year [62]. Apothecia mainly form in the trunk and dead branches of adult trees and small plants [75]. In the second year, G. abietina progresses down along the branch where it causes cankers [76]. Apothecia are gregarious, superficial, and up to 1 mm in diameter. The hymenium is cream-coloured, while the receptacle is dark brown or black [68]. Asci are not operculated; they have eight ascospores and measure 100–120 × 8–10 microns [65]. The fungus has hyaline ascospores that are ellipsoidal, sometimes slightly curved and have rounded ends. Mature ascospores have three septa and measure 15–22 × 3–5 microns. They are hyaline, filiform, and have septate paraphyses [11, 71].
Infection Process and Dispersion of the Fungus
The main mode of infection of G. abietina is through the conidia produced by the anamorph or asexual stage. In humid conditions, the pycnidia release the conidia, which are dispersed by rain splashes [12, 68] over short distances of no more than 4–6 m [77], where they infect neighbouring branches through buds and needles. Spores germinate on the surface and generate mycelial tubes that penetrate the plant through stomata [63, 78], and they begin to form a mycelium inside the plant. To continue the infection and to spread inside, the fungus must be able to penetrate the cell walls. For this purpose, it forms an extracellular sheath containing chemical compounds such as chitin, galactose, proteins, lipids, and polygalacturonic acids [79, 80]. The fungus produces enzymes such as cellulase, exoglucanases, xylanase, and polygalacturonase to enable it to degrade cellulose during the shoot invasion [81,82,83,84], which appears essential during this process of colonisation. In response to G. abietina infections, some induced defence mechanisms have been reported to occur in several pine species and tissues. These include both physical alterations such as the sporadic formation of traumatic resin channels in the xylem and ligno-suberised barriers, and chemical reactions such as the accumulation of large quantities of phenols in cell walls and changes in polyamines [78, 85].
Infection can also occur in conditions of high humidity through the sexual spores (the ascospores), which are windborne and can be dispersed long distances. However, although apothecia are rare in G. abietina (the A biotype) [58], they have been confirmed to be present in northern Europe [53, 86] (also relatively infrequent), and therefore, the dispersal strategy differs from that of type B. The movement of infected seeds and Christmas trees may enable the disease to be dispersed over long distances [65].
Impact of Climate Change and Other Factors
The climatic conditions most conducive to the establishment and outbreak of G. abietina and that enhance the infection include long periods of cold, high humidity during the spring and summer [1, 87, 88]. Climate change is predicted to influence forest disturbances affecting the health and resilience of forest ecosystems worldwide [89]. This will mainly be because drought and other abiotic stressors exacerbated by climate change will probably imply more frequent and intense outbreaks of forest diseases caused by forest pathogens. However, predictions about the future impacts of these diseases are uncertain, partly because the effects of climate change on host-pathogen interactions are complex [90, 91]. For example, the general pattern of future change in annual precipitation over Europe is for widespread increases in northern Europe, smaller decreases across southern Europe, and small or ambiguous changes in central Europe [92]. Therefore, in some regions, for instance, in Northern Sweden or Finland, climate change is predicted to increase suitable conditions for the development of G. abietina—i.e. mild winters followed by cool, wet summers—increasing the risk of epidemic outbreaks in this area [93]. On the other hand, in southern Europe, the most probable climate change scenarios include dry periods in combination with higher temperatures [92] and although trees would be weakened, dispersion of the pathogen could be affected, since its growth is limited by temperature and humidity [94]. However, there remains some uncertainty about the limitations of the spread and establishment of G. abietina, since it has been reported within a wide variety of climatic and ecological conditions [11].
The microclimate and topography of the area may affect the fitness of trees and subsequently also influence the proliferation of G. abietina [73, 77]. The seed origin—and in some cases fertilisation—can also influence the susceptibility of pines [67]. Conversely, high densities of trees in plantations and natural forests can also lead to an increase in the damage produced by G. abietina [95]. Finally, Virtanen et al. (1997) [96] studied the influence of aphids on the damage caused by G. abietina and showed that the presence of the aphid Cinara pinea Mordv. can increase the severity of the disease, while also serving as a vector by transporting the spores.
Control and Management of the Disease
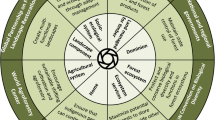
Disease management in forestry requires an understanding of environment, host and pathogen interactions, and pathogen biology and ecology [66], and almost all of the control measures are preventive rather than therapeutic [74]. Classical concepts of plant disease control include avoidance, exclusion, eradication, protection, resistance, and therapy (Table 1) [74]. The measures for managing or controlling disease caused by G. abietina can be broadly summarised in five categories: early detection, silvicultural, chemical, biological control, and host resistance (Fig. 5). In the case of Gremmeniella abietina, the most effective control measure is prevention, especially through avoidance or resistance [118].
Summary of control measures: early detection; silvicultural, chemical, and biological control; and host resistance (created with BioRender.com)
Early Detection of the Pathogen
Preventive measures and early detection of the pathogen are key to controlling the invasion of other territories. Current recommendations in this early detection step include the use of sentinel plantings (plantations, nurseries, or arboreta) that may help predict associated risks to plants [119•]. A major threat of new G. abietina attacks may come with the introduction of the pathogen to new areas with the movement of species in plant trade, such as the North American and/or Asian race in Europe, or the movement of G. abietina within the EU [11]. This risk will be increased by the lack of co-evolution of new putative hosts and the pathogen together with an uncertain level of recovery of the infected stand [120, 121]. In this sense, the definition of new species as emphasised in the first part of this paper could facilitate the implementation of specific measures against the pathogen and legislation in the EU and other territories [11]. Another challenge is the detection of the pathogen in asymptomatic plants. Non-invasive phenotyping approaches, together with the use of high-throughput technologies, will improve the accuracy of detection, prevent the spread of the pathogen in symptomless plants, and reduce the risk of new introductions [122,123,124].
Silvicultural and Cultural Control
Silvicultural methods are aimed at preventing the disease and reducing the progression of the disease once a stand has been infected [74]. Some of the management guidelines for preventing the disease include avoiding the use of susceptible plant species, maintaining the health and vigour of the trees and protecting genetic resources [74, 125]. Other methods include selecting sites far away from infested stands and those unsuitable for plant growth to prevent stress to trees [126, 127]. For instance, previous findings have shown that P. contorta and P. sylvestris plantations in areas originally covered by pure spruce forests are exposed to a high risk of damage by G. abietina [127]. Furthermore, the climatic conditions of the planting sites should not favour dissemination of infection by G. abietina, i.e. cool and wet spring periods and the risk of frost damage [11]. Avoiding introduction of thermophilic species in northern or cold regions, plantations in depressions, and northern slopes in mountainous areas [73•], as well as the development of plantations under conditions of excessively high density [95], is advisable.
Proper stand management should include regular cleaning, thinning, and, in some cases, pruning to avoid shaded, dense, and humid conditions that reduce tree fitness and favour the spread and development of the pathogen [7, 11, 49, 95, 102]. For instance, in Denmark, epidemics in susceptible pine species, especially in P. nigra forests, occur in dense, unthinned stands [88]. In some parts of Quebec, the systematic pruning of low branches of pines was found to be quite effective in the control of the disease [76]. Treatments of this type were carried out on Pinus resinosa and reduced the incidence rate of the disease caused by G. abietina (=European race) from initial values of 67 to 22% [102•]. This control measure is quite effective on pines when most of the infections are still limited to shoots and have not yet reached the trunk, regardless of the Gremmeniella race or biotype. The height of pruning is not restricted to the snow level but is also carried out at the level of highest shoot infections in the crown of trees. The one exception is Pinus banksiana, which is shows some resistance to G. abietina. In natural regeneration of P. banksiana affected by Gremmeniella in North America, pruning is not generally required, as the number of seedlings is very high, and the pathogen only kills the short trees in the snow; the fast-growing pines survive the disease when they reach a height of around 2 m [52, 128]. Finally, as another recommendation, when an infected plantation is harvested, replanting should be delayed for at least two growing seasons [73•], and debris should be burnt or completely removed to minimise the risk of reinfection [129].
Silvicultural measures can also be useful once a stand has become infected. Thus, to limit the spread of the disease, reducing the source of inoculum may be effective. It is therefore useful to understand the life cycle of the pathogen in all locations (i.e. the timing and level of spore dispersion of G. abietina), to enable planning of management actions to prevent the presence of susceptible trees or tissues during the disease spore dissemination periods [130]. Thinning to remove dead or damaged trees is another possible measure to reduce the source and level of inoculum in G. abietina infections. Through such operations, the micro-climatic conditions can be changed, and weakened trees that may become sources of re-infection in the following years can be removed [131]. In some other cases, pruning-infected branches are also recommended, although this should be carried out with caution because it can damage trees and create new infection courts [121]. Although some authors [101, 102] have stated that there is no need to remove pruned branches from the stand if they are left at a distance of at least 60 cm away from healthy branches, several other studies have found re-infections in healthy trees from this infected material by Gremmeniella from North America [100, 102]. Therefore, the removal or burning of infected debris is highly recommended for preventing or minimising re-infections, as the conditions created in the debris piles could greatly enhance spore production [1, 132]. However, if debris is left on the ground but not piled up, pruning will be successful as long as it is done up to the highest infection point; dead pines must be cut down with all their branches removed from the trunk [102•].
Plantations that are severely weakened by G. abietina are also more likely to be attacked by the bark beetle Tomicus piniperda L. [133, 134]. In Sweden, it has been observed that trees that had been affected first by the pathogen had a lower capacity to activate defence mechanisms after a secondary outbreak of T. piniperda [135••]. The secondary damage produced by the insect (or by other opportunistic pests and pathogens) can be reduced by thinning the stand during winter after a severe attack of G. abietina [131]. After 5 years of monitoring following a G. abietina attack in a Scots pine stand, Sikström et al. [136, 137] indicated that only trees with crown transparency over 80% should be removed to prevent damage caused by T. piniperda, unless the percentage of remaining trees was very low, in which case, all of the trees should be removed for sanitary thinning as soon as possible. The remaining, partly damaged trees may recover adequately, as they can develop secondary buds to maintain growth. However, this strategy may fail if the fungus attacks again and damages the newly formed buds and shoots [138]. Recovery of partly damaged trees has been also observed when the environmental conditions following the outbreak limit the occurrence of new epidemics, i.e. high precipitation in summer followed by dry weather [106]. Therefore, forest stands may recover from pathogen outbreaks in a period of approximately 10 years [137••], although considerable growth losses could be reported even in slightly affected pine forests [139••].
Chemical Control
The term fungicide is used in a broad sense for any compound that kills or inactivates fungi. Most of these compounds are chemically synthesised, although some are modified derivatives of naturally occurring compounds [140]. Fungicides are categorised in several ways on the basis of different characteristics. The main classifications are based on the following: (1) antifungal target of the fungicide or mode of action; (2) mobility of the fungicide in the plant: contact or systemic; (3) role in protection: preventive or curative; (4) breadth of activity: single or multi-site, and (5) type of chemical: organic or inorganic [97, 140, 141]. Chemical control may have deleterious impacts on biodiversity and potential for the development of pathogen resistance; the application of fungicides to control G. abietina in the field should therefore be avoided, since many are banned or restricted in many territories such as Europe [16] or North America (Environmental Protection Agency). Some restrictions may be applied when the infestation level is so high that the advantages of application outweigh the above-cited disadvantages [106, 117].
Kohn [142] considered Maneb, a multi-site contact fungicide, to be the best chemical for controlling G. abietina in nurseries. Other authors recommend the application of contact fungicides such as Ziram or Chlorothalonil to prevent dispersion of the pathogen and to reduce the incidence of the pathogen in seedlings [143,144,145]. Smerlis [146] tested several chemical fungicides and recommended application twice, at 2-week intervals. Likewise, Santamaría et al. [117] studied the effect of several fungicides on preventing the growth of Spanish isolates of G. abietina within in vitro experiments and concluded that both Chlorothalonil and Daconil reduced growth of the pathogen at low application doses. Nevertheless, the use of some of these fungicides is no longer approved in the EU [147]. Finally, in Serbia and Montenegro, preliminary research has found that the best results for controlling of the pathogen were obtained by application of copper-based fungicides (e.g. copper oxychloride) twice a year during the critical period of infection [106]. However, application of fungicide should be limited to nurseries and should be performed with caution, since it can mask the symptoms of the disease, resulting in infected asymptomatic plants that spread the pathogen over long distances [11].
Biological Control
Biological control is described as the use of living organisms, naturally derived bioactive compounds, or the induction of natural resistance of the plants to fight against a disease [148]. Biological control agents (BCAs) include bacteria, fungi, nematodes, protozoa, and viruses. They are not usually pathogen-specific, and they combine different modes of action, including the following: (i) mycoparasitism—feeding of one organism on another; (ii) competition—the result of two or more organisms trying to use the same resources; (iii) antibiosis—the inhibition or destruction of one organism by a metabolite produced by another organism; (iv) induction of plant defence system by nonpathogenic fungi; (v) a physical barrier effect produced by the presence of the mycorrhizae; and (vi) hypovirulence—the reduction of the virulence of a pathogen-strain by the presence of a virus [98, 149,150,151].
Fungal endophytes have been identified as BCAs in several other pathosystems [152, 153] and are described as organisms that live inside the plant tissue [154, 155]. If they maintain a beneficial relationship, then they are called mutualists, and if they have a neutral relationship with the plant, then they are commensalists; the lifestyle of most tree endophytes is, however, unknown [155]. When endophytes maintain a detrimental relationship with the plant, they are antagonists and described as pathogens [97]. Biological control with fungal endophytes has already been demonstrated to be effective against G. abietina infections in some experiments. The fungal endophyte Phaeotheca dimorphospora Desrochers and Ouellette inhibited growth of G. abietina colonies, germination of spores, and spread of the pathogen on seedlings of red pine (Pinus resinosa Ait.) [114•]. Regarding Spanish isolates of G. abietina, several endophytes including Trichoderma spp., Aureobasidium pullulans (de Bary & Löwenthal) G. Arnaud, Aureobasidium sp., and Leotiomycete and one unknown fungus referred to as 20.1 reduced or inhibited mycelial growth both in vitro [117] and in vivo in Pinus halepensis seedlings [115]. The efficacy of these water extracts from pure cultures of these fungi was also tested in vitro and in vivo [116, 117] with positive results. The presence of p-hydroxybenzoic acid was reported in some of the extracts and could be responsible for the antagonistic activity observed [116]. The concept of ecosystem microbiome is gaining attention in forest management and restoration. This approach promotes microbial diversity within the tree and soil, since it is known that the microbiome drives essential macro-scale processes in plants and the entire ecosystem, which may boost disease protection [156, 157].
Hypovirulence (i.e. lower virulence of the pathogen) is caused when the fungal isolate is infected by viruses, also called mycoviruses, which mainly have genomes with single-stranded (ss) RNA or double-stranded (ds) RNA and are obligate parasites of fungi [158, 159]. The use of hypovirulence as a biocontrol strategy can be only implemented if two requirements are met: firstly, if any of the mycovirus can reduce the fitness of the pathogenic fungus, and secondly, if the virus can be transmitted efficiently enough to be maintained in a high proportion of the pathogen population [98, 160]. So far, eight mycoviruses belonging to different families have been described in G. abietina [161]. Studies on hypovirulence have not been completely satisfactory as although differences in growth rates have been found between mycovirus-free isolates, no differences in virulence between both types were observed [162]. However, the presence of Gremmeniella abietina RNA virus 6 (GaRV6) was shown to have a negative effect on the mycelial growth rate of G. abietina in vitro [163].
Host Resistance
Disease resistance is one of the most important factors contributing to the long-term stability of plantations. Resistance to pathogens can involve several different processes: avoidance or inhibition, killing the threat, limiting spread, or host repair [164]. Other traits permit the plant to become infected but not severely damaged, and such plants are considered tolerant to the disease [74]. While resistance can be costly to the host, tolerance can provide an evolutionarily stable strategy for coping with moderate pathogen pressure [121]. The improvement in host resistance to pathogens is brought about by breeding and using resistant varieties or provenances [12, 97].
Resistance to infection by G. abietina is likely to be related to (i) production of ligno-suberised tissues that help the tree to compartmentalise the invaded tissues and (ii) secretion of molecules capable of degrading or altering the fibrillar matrix of the extracellular sheath of the pathogen that contains chitin, galactose, proteins, lipids, and polygalacturonic acids [85, 165,166,167]. In some studies, P. banksiana and P. contorta were found to be resistant to the pathogen [85, 165, 166]. Furthermore, some countries such as Canada, Germany, Norway, Sweden, and the USA have developed breeding programmes to identify and select species and individuals, such as P. banksiana, P. sylvestris, and P. nigra that are resistant to the disease [3, 48, 75, 168,169,170]. In Spain, some pine species have also been tested for susceptibility, with P. halepensis being the most susceptible and P. nigra and P. pinaster Ait. the most resistant [112].
Within certain tree species, some provenances or varieties will show different degrees of resistance to the disease. In eastern Canada, the susceptibility of 41 Pinus banksiana provenances to Gremmeniella from North America was tested [111]. None of the provenances tested were resistant to the disease; nevertheless, 11 provenances, all from the most northeastern part of the Quebec province, were classified as the least susceptible. Hansson [109•] tested the susceptibility of several provenances of P. sylvestris, P. contorta, and Picea abies to G. abietina in Sweden. The frequency of affected seedlings in this study differed depending on the latitude of origin and in some cases was related to the non-lignified percentage of the stem. Therefore, the risk to G. abietina damage is related to the relative provenance, i.e. to the distance between the origins of the seed for planting to the location of the planting site. During the Gremmeniella epidemic in the 1980s in Finland, where damage was most severe in forests, the risk of damage was higher in provenances from south of the regeneration area [171]. Some differences in susceptibility to isolates of G. abietina depending on the elevation of the origin of the provenances were also observed in Spain [113] in P. halepensis. However, the choice of hardy provenances is not always a guarantee of success. During the 1987–92 outbreak of Gremmeniella (STT type) in Sweden, the most northern and hardy provenances of P. contorta were also severely damaged [172].
Conclusions
Gremmeniella abietina is an invasive fungal pathogen that represents a major threat to the health of coniferous forests and plantations worldwide. Although forest stands may recover from pathogen outbreaks in a period of approximately 10 years [137••], considerable loss of growth may occur, even in slightly affected pine forests [139••], and the attack can lead to tree decline and death from secondary biotic factors [135••]. Forest disturbances such as pest and disease outbreaks are expected to be exacerbated by the effects of climate change on forest ecosystems worldwide [89, 90] although the exact impact on all host-pathogen interactions remains unclear [173]. In the case of G. abietina, climate change is expected to have different effects in different regions where there is a risk of epidemics occurring. In some areas, it is likely to increase conditions conducive to the development of the fungus, while in others, it will probably limit the spread and growth of the fungus because of high temperatures and low humidity. The complexity of these host-pathogen-environment dynamics makes the outcome of the cascade of interactions precipitated by climate change uncertain [173]. Four categories of measures for managing forest diseases under a changing climate have been recommended: monitoring, forecasting, planning, and use of mitigating strategies [91].
Preventive measures and early detection of the pathogen are key to controlling the invasion of other territories. The introduction of the pathogen to new areas with the movement of species in plant trade and the detection of the pathogen in asymptomatic plants are challenges that should be addressed. The identification of new species and implementation of new technological approaches will improve the accuracy of detection and facilitate legislation with concrete measures to control the spread of the pathogen [122,123,124].
Finally, maintaining healthy and biodiverse ecosystems seems to be a key point of prevention [123]. A holistic approach that combines silvicultural methods, biological control, and host resistance seems to have some effect in the fight against the disease, although with different results depending on the tree species. Using suitable provenances of forest regeneration material is a very important preventive measure: as long as the pine forests are well adapted to their environments, the risk of Gremmeniella epidemic will remain low. However, the severe economic and environmental consequences of the outbreaks, the possibility of re-infection and the unpredictable impacts of climate change must be addressed. Considering the ecosystem as a whole, the tree as a multitrophic community, understanding the adaptive patterns and mechanisms of plants, pathogens, and their interactions—including the forest microbiome concept—are key elements for mitigating the effect of pathogen spread to prevent new outbreaks in the future [124, 156, 157, 173]. Interdisciplinary collaboration among forest pathologists, managers, microbiologists, tree geneticists, forest owners, and tree nurseries is essential in the search for real solutions.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Donaubauer E. Environmental factors influencing outbreak of Scleroderris lagerbergii Gremmen. Eur J For Pathol. 1972;2:21–5. https://doi.org/10.1111/j.1439-0329.1972.tb00339.x.
Yokota S, Uozumi T, Matsuzaki S. Scleroderris canker of Todo-fir in Hokkaido, Northern Japan. Eur J For Pathol. 1974;4:155–66. https://doi.org/10.1111/j.1439-0329.1974.tb00431.x.
Dorworth CE. Disease problems in intensively managed forests — Scleroderris lagerbergii. Eur J For Pathol. 1974;4:228–32. https://doi.org/10.1111/j.1439-0329.1974.tb00441.x.
Morelet M. The Brunchorstia disease.1. Taxonomy and nomenclature of the pathogen. Eur J For Pathol. 1980;10:268–77.
Barklund P, Rowe J. Gremmeniella abietina (Scleroderris lagerbergii), a primary parasite in a Norway spruce dieback. Eur J For Pathol. 1981;11:97–108. https://doi.org/10.1111/j.1439-0329.1981.tb00075.x.
Kaitera J, Jalkanen R. Disease history of Gremmeniella abietina in a Pinus sylvestris stand. Eur J For Pathol. 1992;22:371–8. https://doi.org/10.1111/j.1439-0329.1992.tb00309.x.
Bergdahl DR, Kelley R, Teillon HB. History of Scleroderris canker in Vermont (1971-1996). Phytopathology. 1996;86:S119.
Santamaría O, Pajares JAJ, Diez JJ. First report of Gremmeniella abietina on Pinus halepensis in Spain. Plant Pathol. 2003;52:425. https://doi.org/10.1046/j.1365-3059.2003.00847.x.
Capretti P, Santini A, Solheim H. Branch and tip blights. In: Gonthier P, Nicolotti G, editors. Infectious forest diseases. Wallingford, UK; Boston, MA: CABI; 2013. p. 420–35. https://doi.org/10.1079/9781780640402.0420.
• Wulff S, Hansson P, Witzell J. The applicability of national forest inventories for estimating forest damage outbreaks – experiences from a Gremmeniella outbreak in Sweden. Can J For Res. 2006;36:2605–13. https://doi.org/10.1139/x06-148. Interesting study on the use of large forest inventories for the estimation of the pathogen damage.
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen-Schmutz K, Gilioli G, Gregoire J-C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, et al. Scientific opinion on the pest categorisation of Gremmeniella abietina. EFSA J. 2017;15(11):5030. https://doi.org/10.2903/j.efsa.2017.5030.
Butin HH. Tree diseases and disorders: causes, biology, and control in forest and amenity trees. Oxford University Press; 1995.
Nef L, Perrin R, Commission E. Damaging agents in European forest nurseries. In: Practical handbook. Office for Official Publications of the European Communities: Stationery Office [distributor]. Luxembourg; 1999.
Hudler GW, Neal BG. Scleroderris canker in New York State: attempts to justify and cope with regulatory action. Eur J For Pathol. 1990;20:106–12. https://doi.org/10.1111/j.1439-0329.1990.tb01278.x.
Fragniere Y, Sonnenwyl V, Clement B, Kozlowski G. Large-scale historical afforestation failure with Pinus cembra in the Swiss Prealps. New For. 2022;53:533–53. https://doi.org/10.1007/s11056-021-09871-0.
Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. 2009. http://data.europa.eu/eli/dir/2009/128/oj.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) No 228/2013, (EU) No 652/2014 and (EU) No 1143/2014 of the European Parliament and of the Coun. 2016. http://data.europa.eu/eli/reg/2016/2031/oj.
Farr DF, Rossman AY. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. 2021.
Winter G. Ein Notizblatt für kryptogamische Studien nebst Repertorium für kryptogamische Literatur. Hedwigia. 1884;23:62.
Höhnel F. Mycologische Fragmente. Ann Mycol. 1903;1:522–34.
Brunchorst J. Über eine neue verheerende Krankheit der Schwarzföhre. Bergen Museums Aarsberet. 1888;VI:1–16.
Eriksson J. Fungi parasitici scandinavici. Bot Cent. 1891;47:298.
Höhnel FV. Über Exipulina pinea (Karst.) v Höhn. Fragm zur Mycol. 1915;17:142–3.
Lagerberg T. Granens topptorka. Sven Skogsvardsföreningens Tidskr. 1913;10:173–208.
Van Vloten H, Gremmen J. Studies in the Discomycete genera Crumenula de Not. and Cenangium Fr. Acta Bot Neerl. 1953;2:226–41. https://doi.org/10.1111/j.1438-8677.1953.tb00273.x.
Gremmen J. Some additional notes on Crumenula de Not. and Scleroderris (FR.) de Not. Sydowia. 1955;9:231–2.
Schlaepfer-Bernhard E. Beitrag zur Kenntnis der Discomycetengattungen Godronia, Ascocalyx. Neogodronia und Encoeliopsis. Sydowia. 1969;22:1–56.
Morelet M. Un discomycete inopercule nouveau. Bull Soc Sci Nat Archeol. 1969;183:9.
Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, Seifert KA, et al. The Amsterdam declaration on fungal nomenclature. IMA Fungus. 2011;2:105–11. https://doi.org/10.5598/imafungus.2011.02.01.14.
Wingfield MJ, De Beer ZW, Slippers B, Wingfield BD, Groenewald JZ, Lombard L, et al. One fungus, one name promotes progressive plant pathology. Mol Plant Pathol. 2012;13:604–13. https://doi.org/10.1111/j.1364-3703.2011.00768.x.
Johnston PR, Seifert KA, Stone JK, Rossman AY, Marvanová L. Recommendations on generic names competing for use in Leotiomycetes (Ascomycota). IMA Fungus. 2014;5:91–120. https://doi.org/10.5598/imafungus.2014.05.01.11.
Ettlinger L. Über die Gattung Crumenula sensu Rehm mit besonderer Berücksichtigung des Crumenula-Triebsterbens des Pinus-Arten. Beiträge Kryptogamenflora der Schweiz. 1945;10:1–75. https://doi.org/10.3929/ethz-a-000099021.
Petrini O, Petrini LE, Laflamme G, Ouellette GB. Taxonomic position of Gremmeniella abietina and related species: a reappraisal. Can J Bot. 1989;67:2805–14. https://doi.org/10.1139/b89-360.
Hamelin RC, Bourassa M, Rail J, Dusabenyagasani M, Jacobi V, Laflamme G. PCR detection of Gremmeniella abietina, the causal agent of Scleroderris canker of pine. Mycol Res. 2000;104:527–32. https://doi.org/10.1017/S0953756299002026.
Laflamme G, Smerlis E. Gremmeniella balsamea sp. nov. on balsam fir in Canada. North Am Fungi. 2012;7:1–4. https://doi.org/10.2509/naf2012.007.004.
Morelet M. Notes de mycologie appliquée. Ann la SSNATV. 1995;47:89–94.
Kondo H, Kobayashi T. A new canker disease of loblolly pine, Pinus taeda L., caused by Ascocalyx pinicola sp.nov. J Jap For Soc. 1984;66:60–6. https://doi.org/10.11519/jjfs1953.66.2_60.
Petrini O, Toti L, Petrini E, Heiniger U. Gremmeniella abietina and G. laricina in Europe: characterization and identification of isolates and laboratory strains by soluble protein electrophoresis. Can J Bot. 1990;68:2629–35. https://doi.org/10.1139/b90-332.
Bernier L, Hamelin RC, Ouellette GB. Comparison of ribosomal DNA length and restriction site polymorphisms in Gremmeniella and Ascocalyx isolates. Appl Environ Microbiol. 1994;60(4):1279–86. https://doi.org/10.1128/aem.60.4.1279-1286.1994.
Hamelin RC, Lecours N, Hansson P, Hellgren M, Laflamme G. Genetic differentiation within the European race of Gremmeniella abietina. Mycol Res. 1996;100:49–56. https://doi.org/10.1016/S0953-7562(96)80099-2.
Dorworth CE, Krywienczyk J. Comparisons among isolates of Gremmeniella abietina by means of growth rate, conidia measurement, and immunogenic reaction. Can J Bot. 1975;53:2506–25. https://doi.org/10.1139/b75-276.
Lecours N, Toti L, Sieber TN, Petrini O. Pectic enzyme patterns as a taxonomic tool for the characterization of Gremmeniella spp. isolates. Can J Bot. 1994;72:891–6. https://doi.org/10.1139/b94-114.
Müller MM, Uotila A. The diversity of Gremmeniella abietina var. abietina FAST-profiles. Mycol Res. 1997;101:557–64. https://doi.org/10.1017/S0953756296002894.
Dusabenyagasani M, Lecours N, Hamelin RC. Sequence-tagged sites (STS) for studies of molecular epidemiology of scleroderris canker of conifers. Theor Appl Genet. 1998;97:789–96. https://doi.org/10.1007/s001220050957.
Dusabenyagasani M, Laflamme G, Hamelin RC. Nucleotide polymorphisms in three genes support host and geographic speciation in tree pathogens belonging to Gremmeniella spp. Can J Bot. 2002;80:1151–9. https://doi.org/10.1139/b02-103.
Hantula J, Müller MM. Variation within Gremmeniella abietina in Finland and other countries as determined by random amplified microsatellites (RAMS). Mycol Res. 1997;101:169–75. https://doi.org/10.1017/S0953756296002225.
Laflamme G, Hopkin AA, Harrison KJ. Status of the European race of Scleroderris canker in Canada. For Chron. 1998;74:561–6. https://doi.org/10.5558/tfc74561-4.
Dorworth CE. Influence of inoculum concentration on infection of red pine seedlings by Gremmeniella abietina. Phytopathology. 1979;69:298–300. https://doi.org/10.1094/Phyto-69-298.
Skilling DD, Schneider B, Dasking D. Biology and control of Scleroderris canker in North America. USDA For Serv Res Pap NC-275; 1986. https://doi.org/10.2737/NC-RP-275.
Shilling DD. The development of a more virulent strain of Scleroderris lagerbergii in New York State. Eur J For Pathol. 1977;7:297–302. https://doi.org/10.1111/j.1439-0329.1977.tb00592.x.
Uotila A. Physiological and morphological variation among Finnish Gremmeniella abietina isolates. Commun Instituti For Fenn. 1983:119.
Hellgren M, Högberg N. Ecotypic variation of Gremmeniella abietina in northern Europe: disease patterns reflected by DNA variation. Can J Bot. 1995;1539:1531–9. https://doi.org/10.1139/b95-166.
Uotila A, Hantula J, Vaatanen AK, Hamelin RC. Hybridization between two biotypes of Gremmeniella abietina var. abietina in artificial pairings. For Pathol. 2000;30:211–9. https://doi.org/10.1046/j.1439-0329.2000.00207.x.
Uotila A, Hantula J. The Gremmeniella spp. taxonomy- types, races or species? In: Proceeding IUFRO WP 70202 Foliage, Shoot stems Dis. Czech Republic: Brno and Cerna Hora; 2012. p. 53. This paper emphasises the importance of defining new species inside the Gremmeniella abietina complex.
Botella L, Tuomivirta TT, Kaitera J, Carrasco Navarro V, Diez JJ, Hantula J. Spanish population of Gremmeniella abietina is genetically unique but related to type A in Europe. Fungal Biol. 2010;114:778–89. https://doi.org/10.1016/j.funbio.2010.07.003. In this paper, the authors study the genetic structure of the European G. abietina population.
Uotila A. Variation in uniascus monoascospore cultures of Ascocalyx abietina. Scots pine diseases: proceedings of an international symposium Kórnik, Poland, 16-20, May 1989: joint meeting: 7th European Colloquium of Forest Pathologists and the members of two IUFRO Working Parties S2.05.04 Resistance of pines to Melampsora pinitorqua, S2.06.02 Canker and shoot blight of conifers. Metsäntutkimuslaitoksen Tied. 1990;147 http://urn.fi/URN:ISBN:951-40-1100-7
Botella L, Tuomivirta TT, Hantula J, Diez JJ, Jankovsky L. The European race of Gremmeniella abietina hosts a single species of Gammapartitivirus showing a global distribution and possible recombinant events in its history. Fungal Biol. 2015;119:125–35. https://doi.org/10.1016/j.funbio.2014.12.001.
Kaitera J, Jalkanen R. In vitro growth of Gremmeniella abietina isolates (European race) at different temperatures. Scand J For Res. 1996;11:159–63. https://doi.org/10.1080/02827589609382924.
Uotila A. Genetic variation of Gremmeniella abietina in Finland. In: Jalkanen R, Aalto T, Lahti M-L, editors. Forest pathological research in northern forests with a special reference to abiotic stress factors. Metsäntutkimuslaitoksen tiedonantoja 451 (The Finnish Forest Research Institute. Research Papers 451); 1993. p. 119–22.
Senn J. Tree mortality caused by Gremmeniella abietina in a subalpine afforestation in the central Alps and its relationship with duration of snow cover. Eur J For Pathol. 1999;29:65–74. https://doi.org/10.1046/j.1439-0329.1999.00131.x.
Santamaria O, Alves-Santos FM, Diez JJ. Genetic characterization of Gremmeniella abietina var. abietina isolates from Spain. Plant Pathol. 2005;54:331–8. https://doi.org/10.1111/j.1365-3059.2005.01184.x.
Hellgren M, Barklund P. Studies on the life cycle of Gremmeniella abietina on Scots pine in southern Sweden. Eur J For Pathol. 1992;22:300–11. https://doi.org/10.1111/j.1439-0329.1992.tb00797.x.
Patton RF, Spear RN, Blenis PV. The mode of infection and early stages of colonization of pines by Gremmeniella abietina. Eur J For Pathol. 1984;14:193–202. https://doi.org/10.1111/j.1439-0329.1984.tb00163.x.
Peace TR. Pathology of trees and shrubs. Oxford At The Clarendon Press; 1962.
Phillips DH, Burdekin DA. Diseases of forest and ornamental trees. Palgrave Macmillan London; 1992. https://doi.org/10.1007/978-1-349-10953-1.
Manion PD. Tree disease concepts. Englewood Cliffs, New Jersey; Prentice-Hall;1981. ark:/13960/t9g542884.
Pätilä A, Uotila A. Scleroderris canker and frost damage in fertilized pine stands on an ombrotrophic mire. Scand J For Res. 1990;5:41–8. https://doi.org/10.1080/02827589009382591.
Punithalingam E, Gibson IA. Gremmeniella abietina. CMI descriptions of pathogenic fungi and bacteria, vol. 369. Wallingford, UK: CAB International; 1973.
Lilja A, Poteri M, Petäistö R, Rikala R. Fungal diseases in forest nurseries in Finland. Silva Fenn. 2010;44:525–45. https://doi.org/10.14214/sf.147.
Kaitera J, Hantula J, Jalkanen R. Development of fruiting bodies of large tree type of Gremmeniella abietina var. abietina and timing of infection on Scots pine in northern Finland. Eur J For Pathol. 1997;27:115–24. https://doi.org/10.1111/j.1439-0329.1997.tb01362.x.
Hellgren M, Stenlid J. Scleroderris canker. In: Hansen EM, Lewis KJ, editors. Compend Conifer Dis. St. Paul: American Phytopathological Society Press; 1997.
• Witzell J, Bernhold A, Hansson P. Survival and vitality of Gremmeniella abietina on Pinus sylvestris slash in northern Sweden. For Pathol. 2006;36:406–12. https://doi.org/10.1111/j.1439-0329.2006.00475.x. In this work, the authors investigated the vitality of G. abietina pycnidia in the slash.
• Laflamme G, Rioux D. Two-year survival of Gremmeniella abietina conidia collected on branches left on the ground after pine harvesting. Forests. 2015;6:4055–8. https://doi.org/10.3390/f6114055. This study provides useful information about the survival of the pathogen in natural conditions.
Tainter FH, Baker FA. Principles of forest pathology. Wiley; 1996.
Roll-Hansen F. Scleroderris lagerbergii: resistance and differences in attack between pine species and provenances. Eur J For Pathol. 1972;2:26–39. https://doi.org/10.1111/j.1439-0329.1972.tb00340.x.
Laflamme G. Scleroderris canker on pine. Information Leaflet LFC 3. Quebec: Canadian Forest Service, Canada; 1991.
Uotila A. The effect of climatic factors on the occurrence of Scleroderris canker. Folia For. 1988;721:1–23.
Ylimartimo A, Laflamme G, Simard M, Rioux D. Ultrastructure and cytochemistry of early stages of colonization by Gremmeniella abietina in Pinus resinosa seedlings. Can J Bot. 1997;75:1119–32. https://doi.org/10.1139/b97-123.
Benhamou N, Ouellette GB. Ultrastructural study and cytochemical investigation, by means of enzyme–gold complexes, of the fungus Ascocalyx abietina. Can J Bot. 1987;65:168–81. https://doi.org/10.1139/b87-023.
Benhamou N, Ouellete GB. Ultrastructural characterization of an extracellular fibrillar sheath on cells of Ascocalyx abietina, the Scleroderris canker agent of conifers. Can J Bot. 1987;65:154–67. https://doi.org/10.1139/b87-022.
Petäistö R-L, Talvinen J, Kajander EO. Detection of xylan hydrolyzing activity in culture extracts of Gremmeniella abietina. Eur J For Pathol. 1992;22:349–53. https://doi.org/10.1111/j.1439-0329.1992.tb00306.x.
Petäistö R-L, Rissanen TE, Harvima RJ, Kajander EO. Analysis of the protein pattern of Gremmeniella abietina with special reference to protease activity. Mycologia. 1994;86:242–9. https://doi.org/10.1080/00275514.1994.12026401.
Petäistö R-L, Kajander EO. The ability of Gremmeniella abietina to hydrolyze polygalacturonic acid. Eur J For Pathol. 1993;23:306–13. https://doi.org/10.1111/j.1439-0329.1993.tb00967.x.
Petäistö R-L, Lappi J. Capability of the European and North American race of Gremmeniella abietina to hydrolyse polygalacturonic acid in vitro. Eur J For Pathol. 1996;26:123–32. https://doi.org/10.1111/j.1439-0329.1996.tb00717.x.
Laflamme G, Rioux D, Simard M, Bussières G, Mallett K. Resistance of Pinus contorta to the European race of Gremmeniella abietina. For Pathol. 2006;36:83–96. https://doi.org/10.1111/j.1439-0329.2006.00436.x.
Uotila A, Kurkela T, Tuomivirta T, Hantula J, Kaitera J. Gremmeniella abietina types cannot be distinguished using ascospore morphology. For Pathol. 2006;36:395–405. https://doi.org/10.1111/j.1439-0329.2006.00464.x.
Uotila A, Petäistö R. How do the epidemics of Gremmeniella abietina start. Acta Silv Lign Hung Spec Ed. 2007:147–51.
Thomsen IM. Precipitation and temperature as factors in Gremmeniella abietina epidemics. For Pathol. 2009;39:56–72. https://doi.org/10.1111/j.1439-0329.2008.00561.x.
Pautasso M, Schlegel M, Holdenrieder O. Forest health in a changing world. Microb Ecol. 2015;69:826–42. https://doi.org/10.1007/s00248-014-0545-8.
Sturrock RN. Climate change and forest diseases: using today’s knowledge to address future challenges. For Syst. 2012;21:329–36. https://doi.org/10.5424/fs/2012212-02230.
Sturrock RN, Frankel SJ, Brown AV, Hennon PE, Kliejunas JT, Lewis KJ, et al. Climate change and forest diseases. Plant Pathol. 2011;60:133–49. https://doi.org/10.1111/j.1365-3059.2010.02406.x.
Pörtner HO, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, IPCC, et al. Climate change 2022: impacts, adaptation, and vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; 2022.
Stenlid J, Oliva J. Phenotypic interactions between tree hosts and invasive forest pathogens in the light of globalization and climate change. Philos Trans R Soc B Biol Sci. 2016;371:20150455. https://doi.org/10.1098/rstb.2015.0455.
Santamaría O, Pajares JA, Diez JJ. Physiological and morphological variation of Gremmeniella abietina from Spain. For Pathol. 2004;34:395–405. https://doi.org/10.1111/j.1439-0329.2004.00380.x.
Niemelä P, Lindgren M, Uotila A. The effect of stand density on the susceptibility of Pinus sylvestris to Gremmeniella abietina. Scand J For Res. 1992;7:129–33. https://doi.org/10.1080/02827589209382705.
Virtanen T, Ranta H, Neuvonen S. Shoot-feeding aphids promote development of Gremmeniella abietina, the fungal pathogen causing Scleroderris canker disease in conifers. J Phytopathol. 1997;145:245–51. https://doi.org/10.1111/j.1439-0434.1997.tb00394.x.
Agrios GN. Plant pathology. 4th ed. San Diego, California, USA: Academic Press; 1997.
Ownley BH, Windham MT. Biological control of plant pathogens. In: Trigiano R, Windham M, Windham A, editors. Plant Pathol Concepts Lab Exerc. 2nd ed. Boca Rotan, FL: Taylor and Francis, CRC Press; 2007. https://doi.org/10.1201/b12388.
Mejía LC, Rojas EI, Maynard Z, Van BS, Arnold AE, Hebbar P, et al. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol Control. 2008;46:4–14. https://doi.org/10.1016/j.biocontrol.2008.01.012.
French WJ, Silverborg SB. Scleroderris canker of red pine in New York state plantations. Plant Dis Report. 1967;51:108–9.
Bergdahl DR, Ward TM. Pruning as a silvicultural tool in the management of Pinus resinosa infected with Gremmeniella abietina. In: Manion PD, editor. Scleroderris canker of conifers, vol. 13. Springer, Dordrecht: Forestry Sciences; 1984. https://doi.org/10.1007/978-94-009-6107-4_29.
• Laflamme G. Traitement réussi d’une plantation de pins rouges affectée par le Gremmeniella abietina, race européenne. Phytoprotection. 1999;80:55–64. https://doi.org/10.7202/706180ar. Good example of successful control of the pathogen by silvicultural approaches.
Ericsson A, Lindgren A, Mattsson A. Effects of cold storage and planting period on subsequent growth, starch and nitrogen content in Scots pine (Pinus sylvestris) and Norway spruce (Picea abies) seedlings. Stud For Suec. 1983;165:1–17. http://urn.kb.se/resolve?urn=urn:nbn:se:slu:epsilon-9-33
Hansson P, Karlman M. Survival, height and health status of 20-year-old Pinus sylvestris and Pinus contorta after different scarification treatments in a harsh boreal climate. Scand J For Res. 1997;12:340–50. https://doi.org/10.1080/02827589709355421.
Kujala V. Uber die Kleinpilze der Koniferen in Finnland. Commun Inst For Fenn. 1950;38:121. http://urn.fi/URN:NBN:fi-metla-201207171070
Karadžić D, Milanović S. Gremmeniella abietina (Lagerb.) Morelet : distribution in Serbia and Montenegro, significance and control. Bull Fac For. 2008;98:107–16. https://doi.org/10.2298/GSF0898107K.
Wulff S, Walheim M. Gremmeniella abietina: uppträdande i Sverige. Resultat från Riksskogstaxeringen och skogsskadeinventeringen 2002. Umeå.
Skilling DD. The role of silviculture in control of Scleroderris canker. USA: Heal For Heal world Soc Am For. Bethesda; 1988. p. 178–81.
• Hansson P. Susceptibility of different provenances of Pinus sylvestris, Pinus contorta and Picea abies to Gremmeniella abietina. Eur J Plant Pathol. 1998;28:21–32. https://doi.org/10.1111/j.1439-0329.1998.tb01162.x. This work studied the pattern of decreasing damage by G. abietina with the increasing latitude of origin of P. sylvestris provenances.
Skilling DD, Riemenschneider DE. Screening conifers for resistance to Gremmeniella abietina. In: Manion PD, editor. Scleroderris canker of conifers. Forestry Sciences, vol. 13. Dordrecht: Springer; 1984. https://doi.org/10.1007/978-94-009-6107-4_32.
Bussières G, Dessureault M, Laflamme G. Susceptibility of Jack pine from Quebec seed source to Scleroderris canker. In: Kurkela T, Siwecki R, editors. Bull Finnish For Res Inst 360 Proceeding an Int Symp Kornik. Poland; 1990.
Santamaría O, Pando V, Diez JJ. Susceptibility of six pine species to Gremmeniella abietina isolates from Spain. For Pathol. 2006;36:349–59. https://doi.org/10.1111/j.1439-0329.2006.00463.x.
Romeralo C, Witzell J, Diez JJ. Aleppo pine provenances vary in susceptibility and secondary chemical response to Gremmeniella abietina infection. Plant Pathol. 2016;65:664–72. https://doi.org/10.1111/ppa.12452.
• Yang D, Laflamme G, Bernier L, Dessureault M. Phaeotheca dimorphospora as a potential biocontrol agent for shoot blight caused by Gremmeniella abietina. Can. J Plant Pathol. 1995;17:7–12. https://doi.org/10.1080/07060669509500713. This work represents one of the first approaches on the biological control of the pathogen.
Romeralo C, Santamaría O, Pando V, Diez JJ. Fungal endophytes reduce necrosis length produced by Gremmeniella abietina in Pinus halepensis seedlings. Biol Control. 2015;80:30–9. https://doi.org/10.1016/j.biocontrol.2014.09.010.
Romeralo C, Witzell J, Romeralo-Tapia R, Botella L, Diez JJ. Antagonistic activity of fungal endophyte filtrates against Gremmeniella abietina infections on Aleppo pine seedlings. Eur J Plant Pathol. 2015;143:691–704. https://doi.org/10.1007/s10658-015-0719-3.
Santamaría O, Tejerina L, Pajares JA, Diez JJ. Effects of associated fungi Sclerophoma pythiophila and Cenangium ferruginosum on Gremmeniella abietina dieback in Spain. For Pathol. 2007;37:121–8. https://doi.org/10.1111/j.1439-0329.2007.00486.x.
Bernhold A. Management of Pinus sylvestris stands infected by Gremmeniella abietina. Doctoral Thesis, Swedish University of Agricultural Sciences; 2008. https://res.slu.se/id/publ/17880
• Morales-Rodríguez C, Anslan S, Auger-Rozenberg MA, Augustin S, Baranchikov Y, Bellahirech A, et al. Forewarned is forearmed: harmonized approaches for early detection of potentially invasive pests and pathogens in sentinel plantings. NeoBiota. 2019:95–123. https://doi.org/10.3897/neobiota.47.34276. An important review highlighting tools for the early detection of putative invasive forest pest and pathogens.
Ennos RA. Resilience of forests to pathogens: an evolutionary ecology perspective. Forestry. 2014;88:41–52. https://doi.org/10.1093/forestry/cpu048.
Oliva J, Boberg JB, Hopkins AJM, Stenlid J. Concepts of epidemiology of forest diseases. In: Gonthier P, Nicolotti G, editors. Infectious forest diseases. Wallingford, UK; Boston, MA: CABI; 2013. p. 1–28. https://doi.org/10.1079/9781780640402.0001.
Garbelotto M, Pautasso M. Impacts of exotic forest pathogens on Mediterranean ecosystems: four case studies. Eur J Plant Pathol. 2011;133:101–16. https://doi.org/10.1007/s10658-011-9928-6.
Pautasso M, Döring TF, Garbelotto M, Pellis L, Jeger MJ. Impacts of climate change on plant diseases—opinions and trends. Eur J Plant Pathol. 2012;133:295–313. https://doi.org/10.1007/s10658-012-9936-1.
Desprez-Loustau M-L, Aguayo J, Dutech C, Hayden KJ, Husson C, Jakushkin B, et al. An evolutionary ecology perspective to address forest pathology challenges of today and tomorrow. Ann For Sci. 2016;73:45–67. https://doi.org/10.1007/s13595-015-0487-4.
Zeglen S, Pronos J, Merler H. Silvicultural management of white pines in western North America. For Pathol. 2010;40:347–68. https://doi.org/10.1111/j.1439-0329.2010.00662.x.
Nevalainen S. Gremmeniella abietina in Finnish Pinus sylvestris stands in 1986-1992: a study based on the national forest inventory. Scand J For Res. 1999;14:111–20. https://doi.org/10.1080/02827589950152836.
Witzell J, Karlman M. Importance of site type and tree species on disease incidence of Gremmeniella abietina in areas with a harsh climate in Northern Sweden. Scand J For Res. 2000;15:202–9. https://doi.org/10.1080/028275800750015019.
Laflamme G. Natural selection of Pinus banksiana regeneration through increase inoculum of G. abietina, North American race. Acta Silv Lign Hung Spec Ed. 2007;275
Bernhold A, Witzell J, Hansson P. Effect of slash removal on Gremmeniella abietina incidence on Pinus sylvestris after clear-cutting in northern Sweden. Scand J For Res. 2006;21:489–95. https://doi.org/10.1080/02827580601090176.
Petäistö R, Heinonen J. Conidial dispersal of Gremmeniella abietina: climatic and microclimatic factors. For Pathol. 2003;33:363–73. https://doi.org/10.1111/j.1439-0329.2003.00335.x.
Uotila A, Uitamo J. The effect of thinning on the recovery of Scots pine stands suffering from Scleroderris canker. Shoot Dis conifers Proc a IUFRO Work party, Garpenberg, Sweden, 10-15 June 1991 Ed by Barklund, P, Livsey, S, Karlman, M Stephan, R Swedish Univ Agric Sci Uppsala. 1993. pp. 31-35.
Dorworth CE. Longevity of Scleroderris lagerbergii Gremmen in pine slash. Can For Serv Bi-Monthly Res Notes. 1972;28 https://eurekamag.com/research/000/131/000131021.php
Kaitera J, Jalkanen R. Old and fresh Gremmeniella abietina damage on Scots pine in eastern Lapland in 1992. Silva Fenn. 1994;28:107–13. http://hdl.handle.net/1975/9166
Sikström U, Jansson G, Weslien J. Predicting the mortality of Pinus sylvestris attacked by Gremmeniella abietina and occurrence of Tomicus piniperda colonization. Can J For Res. 2005;35:860–7. https://doi.org/10.1139/x05-012.
Oliva J, Stenlid J, Grönkvist-Wichmann L, Wahlström K, Jonsson M, Drobyshev I, et al. Pathogen-induced defoliation of Pinus sylvestris leads to tree decline and death from secondary biotic factors. For Ecol Manag. 2016;379:273–80. https://doi.org/10.1016/j.foreco.2016.08.011. This study shows evidence of pathogen infection as a predisposing factor of decline by secondary factors.
Sikström U, Jacobson S, Pettersson F, Weslien J. Crown transparency, tree mortality and stem growth of Pinus sylvestris, and colonization of Tomicus piniperda after an outbreak of Gremmeniella abietina. For Ecol Manag. 2011;262:2108–19. https://doi.org/10.1016/j.foreco.2011.07.034.
Sikström U, Jacobson S, Pettersson F. Recovery of crown transparency and stem growth of Pinus sylvestris after infestation by Gremmeniella abietina. For Ecol Manag. 2017;392:154–63. https://doi.org/10.1016/j.foreco.2017.02.044. This study highlights the importance of undertaking sanitary cuts as soon as possible after an infestation.
Søgaard G, Solheim H, Johnsen Ø. Secondary buds in Scots pine trees infested with Gremmeniella abietina. Trees. 2007;21:191–9. https://doi.org/10.1007/s00468-006-0111-1.
Wang X, Stenström E, Boberg J, Ols C, Drobyshev I. Outbreaks of Gremmeniella abietina cause considerable decline in stem growth of surviving Scots pine trees. Dendrochronologia. 2017;44:39–47. https://doi.org/10.1016/j.dendro.2017.03.006. This study evaluates the impacts of a G. abietina outbreak on the stem growth of the surviving Scots pine and Norway spruce trees in Sweden.
Deacon JW. Fungal biology. Malden: Blackwell; 2006.
Hirooka T, Ishii H. Chemical control of plant diseases. J Gen Plant Pathol. 2013;79:390–401. https://doi.org/10.1007/s10327-013-0470-6.
Kohh E. Om tallens gren- och granens topptorka och dess bekämpning. Skogen. 1964;51:200–3.
Hopkin AA, McKenney DW. The distribution and significance of Scleroderris disease in Ontario. Nat Resour Canada, Can For Serv. 1995:7–11.
Skilling DD, Waddell CD. Control of Scleroderris canker by fungicide sprays. Plant Dis. 1970;54:663–5.
Dorworth CE. Disease of conifers incited by Scleroderris lagerbergii Gremmen: a review and analysis. Can For Serv Publ. 1971;1289:46.
Smerlis E. Evaluation of fungicides for control of Gremmeniella abietina IV. Results of 1979 and 1980 field assays. Inf Rep LAU-X-58 Can For Serv Rech For des Laurentides; 1983.
Regulation (EC) No 1107/2009 Commission implementing regulation (EU) No 540/2011. 2009. http://data.europa.eu/eli/reg_impl/2011/540/oj.
Talibi I, Boubaker H, Boudyach EH, Aoumar AB, A. Alternative methods for the control of postharvest citrus diseases. J Appl Microbiol. 2014;117:1–17. https://doi.org/10.1111/jam.12495.
Schoeman MW, Webber JF, Dickinson DJ. The development of ideas in biological control applied to forest products. Int Biodeterior Biodegrad. 1999;43:109–23. https://doi.org/10.1016/S0964-8305(99)00037-2.
Alabouvette C, Olivain C, Steinberg C. Biological control of plant diseases: the European situation. Eur J Plant Pathol. 2006;114:329–41. https://doi.org/10.1007/s10658-005-0233-0.
Heydari A, Pessarakli M. A review on biological control of fungal plant pathogens using microbial antagonists. J Biol Sci. 2010;10:273–90. https://doi.org/10.3923/jbs.2010.273.290.
Santamaría O, Smith DR, Stanosz GR. Interaction between Diplodia pinea or Diplodia scrobiculata and fungal endophytes isolated from pine shoots. Can J For Res. 2012;42:1819–26. https://doi.org/10.1139/x2012-132.
Rodrigo S, Santamaria O, Halecker S, Lledó S, Stadler M. Antagonism between Byssochlamys spectabilis (anamorph Paecilomyces variotii) and plant pathogens: involvement of the bioactive compounds produced by the endophyte. Ann Appl Biol. 2017;171:464–76. https://doi.org/10.1111/aab.12388.
Arnold AE. Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biol Rev. 2007;21:51–66. https://doi.org/10.1016/j.fbr.2007.05.003.
Sieber TN. Endophytic fungi in forest trees: are they mutualists? Fungal Biol Rev. 2007;21:75–89. https://doi.org/10.1016/j.fbr.2007.05.004.
Averill C, Anthony MA, Baldrian P, Finkbeiner F, van den Hoogen J, Kiers T, et al. Defending Earth’s terrestrial microbiome. Nat Microbiol. 2022;7:1717–25. https://doi.org/10.1038/s41564-022-01228-3.
Busby PE, Newcombe G, Neat AS, Averill C. Facilitating reforestation through the plant microbiome: perspectives from the phyllosphere. Annu Rev Phytopathol. 2022;60:337–56. https://doi.org/10.1146/annurev-phyto-021320-010717.
Ghabrial SA, Suzuki N. Viruses of plant pathogenic fungi. Annu Rev Phytopathol. 2009;47:353–84. https://doi.org/10.1146/annurev-phyto-080508-081932.
Pearson MN, Beever RE, Boine B, Arthur K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol. 2009;10:115–28. https://doi.org/10.1111/j.1364-3703.2008.00503.x.
McCabe PM, Pfeiffer P, Van Alfen NK. The influence of dsRNA viruses on the biology of plant pathogenic fungi. Trends Microbiol. 1999;7:377–81. https://doi.org/10.1016/S0966-842X(99)01568-1.
Botella L, Hantula J. Description, distribution, and relevance of viruses of the forest pathogen Gremmeniella abietina. Viruses. 2018:10–654. https://doi.org/10.3390/v10110654.
Romeralo C, Botella L, Santamaria O, Diez J. Effect of putative mitoviruses on in vitro growth of Gremmeniella abietina isolates under different laboratory conditions. For Syst. 2012;21:515–25. https://doi.org/10.5424/fs/2012213-02266.
Botella L, Dvořák M, Capretti P, Luchi N. Effect of temperature on GaRV6 accumulation and its fungal host, the conifer pathogen Gremmeniella abietina. For Pathol. 2017;47:e12291. https://doi.org/10.1111/efp.12291.
Telford A, Cavers S, Ennos RA, Cottrell JE. Can we protect forests by harnessing variation in resistance to pests and pathogens? Forestry. 2014;88:3–12. https://doi.org/10.1093/forestry/cpu012.
Simard M, Rioux D, Laflamme G. Formation of ligno-suberized tissues in jack pine resistant to the European race of Gremmeniella abietina. Phytopathology. 2001;91:1128–40. https://doi.org/10.1094/PHYTO.2001.91.12.1128.
Bernhold A, Hansson P, Rioux D, Simard M, Laflamme G. Resistance to Gremmeniella abietina (European race, large tree type) in introduced Pinus contorta and native Pinus sylvestris in Sweden. Can J For Res. 2009;39:89–96. https://doi.org/10.1139/X08-157.
Simard M, Laflamme G, Rioux D. Enzymatic interactions between Gremmeniella abietina var. abietina, European race, and two resistant hosts, Pinus banksiana and P. contorta. For Pathol. 2013;43:29–41. https://doi.org/10.1111/j.1439-0329.2012.00790.x.
Dietrichson J. Provenance and resistance to Scleroderris lagerbergii Gremmen (Crumenula abietina Lagerb.). The international Scots pine provenance experiment of 1938 at Matrand. 1968. Medd Nor ske Skogforsoeksves. 25:395–410.
Teich AH, Smerlis E. Jack pine resistance to Scleroderris lagerbergii. Can For Serv Bi-mon Res Notes. 1969;25(6):47.
Laflamme G, Blais R. Resistance of Pinus banksiana to the European race of Gremmeniella abietina. Phytoprotection. 2000;81:49–55. https://doi.org/10.7202/706199ar.
Kallio T, Häkkinen R, Heinonen J. An outbreak of Gremmeniella abietina in central Finland. Eur J For Pathol. 1985;15:216–23. https://doi.org/10.1111/j.1439-0329.1985.tb00888.x.
Karlman M. Risks associated with the introduction of Pinus contorta in northern Sweden with respect to pathogens. For Ecol Manag. 2001;141:97–105. https://doi.org/10.1016/S0378-1127(00)00492-8.
Burdon JJ, Zhan J. Climate change and disease in plant communities. PLOS Biol. 2020;18(11):e3000949. https://doi.org/10.1371/journal.pbio.3000949.
Acknowledgements
The authors are thankful to the reviewers for their useful and constructive comments.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The study was financed by the projects of the Spanish Ministry of Science and Technology AGL2005-02141/FOR and AGL2008-03622. We also thank the University of Valladolid, the COST action FA110, the government of Canada, and the EVOLTREE group for economic support. L.B. has been supported by the project Phytophthora Research Centre Reg. No. CZ.02.1.01/0.0/0.0/15_003/0000453, which was co-financed by the European Regional Development Fund. Prof. Diez received funding from the projects VA208P20 funded by Junta Castilla y León (JCYL, Spain), co-financed by EU budget (JD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not include any studies with human or animal subjects performed by any of the authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the on Topical Collection on Forest Pathology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Romeralo, C., Botella, L., Santamaría, O. et al. Gremmeniella abietina: a Loser in the Warmer World or Still a Threat to Forestry?. Curr. For. Rep. 9, 332–349 (2023). https://doi.org/10.1007/s40725-023-00193-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-023-00193-2