Welcome to the
Host-Microbiota Interactions and Clostridia Research Group
The Daniel Paredes-Sabja Lab
Department of Biology
Texas A&M University
What do we study? Clostridioides difficile-host interactions, C. difficile spore surface assembly and therapeutic development.
Clostridioides difficile is a Gram positive, strictly anaerobic, spore-forming bacterium that causes C. difficile infections (CDI) and is considered as the most frequent hospital-acquired pathogen. In the US alone, ~500,000 patients per year suffer from CDI with mortality rates that reach ~8% of total CDI-patients, causing major economic burden to the United States Health Care System. Treatment of CDI normally involves antibiotic therapy (vancomycin or fidaxomicin), both of which resolve a first episode of CDI in ~95% of the cases. However, a major clinical challenge in CDI management is the elevated rates of recurrence of CDI (R-CDI), affecting 20-30 % of the patients with CDI, which will experiment a recurrence of the disease with aggravated symptoms. Antibiotic usage is the major cause for decreased resistance to CDI establishment. A continued disrupted intestinal microbiota community has been linked to a lack of protection against recurrence of CDI. Fecal-microbiota transplant (FMT) is an accepted treatment for recurrent CDI with reported efficacy of over 90%, however, there are many potential risks associated to the transfer of live microorganisms to sick patients, which include transfer of multidrug-resistant microorganisms, development of inflammatory bowel disease, cancer, obesity and autoimmune disease.
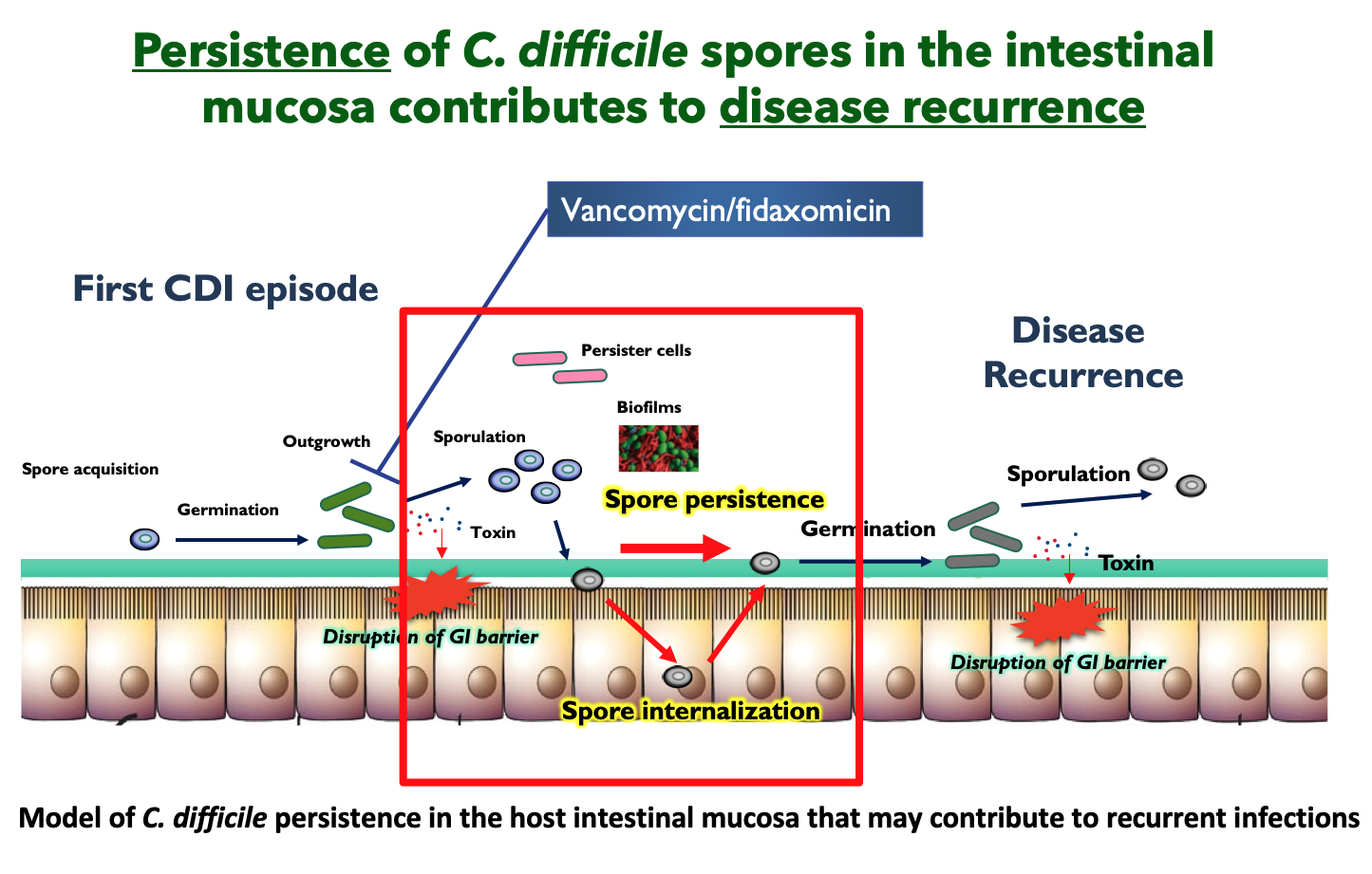
Intestinal dysbiosis results in increased levels of germinants (e.g., taurocholate) that trigger spore-germination of newly-ingested / pre-existing C. difficile spores in the GI tract. In the absence of the microbiota-mediated protective barrier, the resulting vegetative cells colonize and secrete both major virulence factors, toxin A (TcdA) and B (TcdB), responsible for the clinical manifestation of the disease, induce pro-inflammatory cytokines, disruption of tight junctions, detachment of intestinal epithelial cells (IECs), and loss of transepithelial barrier. C. difficile also initiates a sporulation pathway that leads to the production of new metabolically dormant spores in the host’s intestine. Given the intrinsic resistance of these spores to antibiotics and the host´s immune system, which facilitates their persistence in the luminal environment, is that C. difficile spores are considered the main virulence factor linked to disease recurrence. However, the mechanisms employed by C. difficile (spores) to persist in the GI tract during infection-treatment to cause recurrent disease remain elusive. Given that C. difficile is an urgent threat to human health, our group is addressing several independent, and complementary research avenues:
- Mechanisms of C. difficile persistence in the intestinal mucosa. During the infection, C. difficile initiates a sporulation cycle that leads to the formation of new dormant spores in the host, which are key for the recurrence and transmission of the disease. However, the mechanisms that underline C. difficile spore-persistence remain unclear. Our aim is to elucidate how C. difficile spores interact with the intestinal mucosa and persist during the infection. To accomplish this aim, we blend expertise from bacterial genetics, cellular microbiology, cellular biology, animal models and advance imaging techniques.
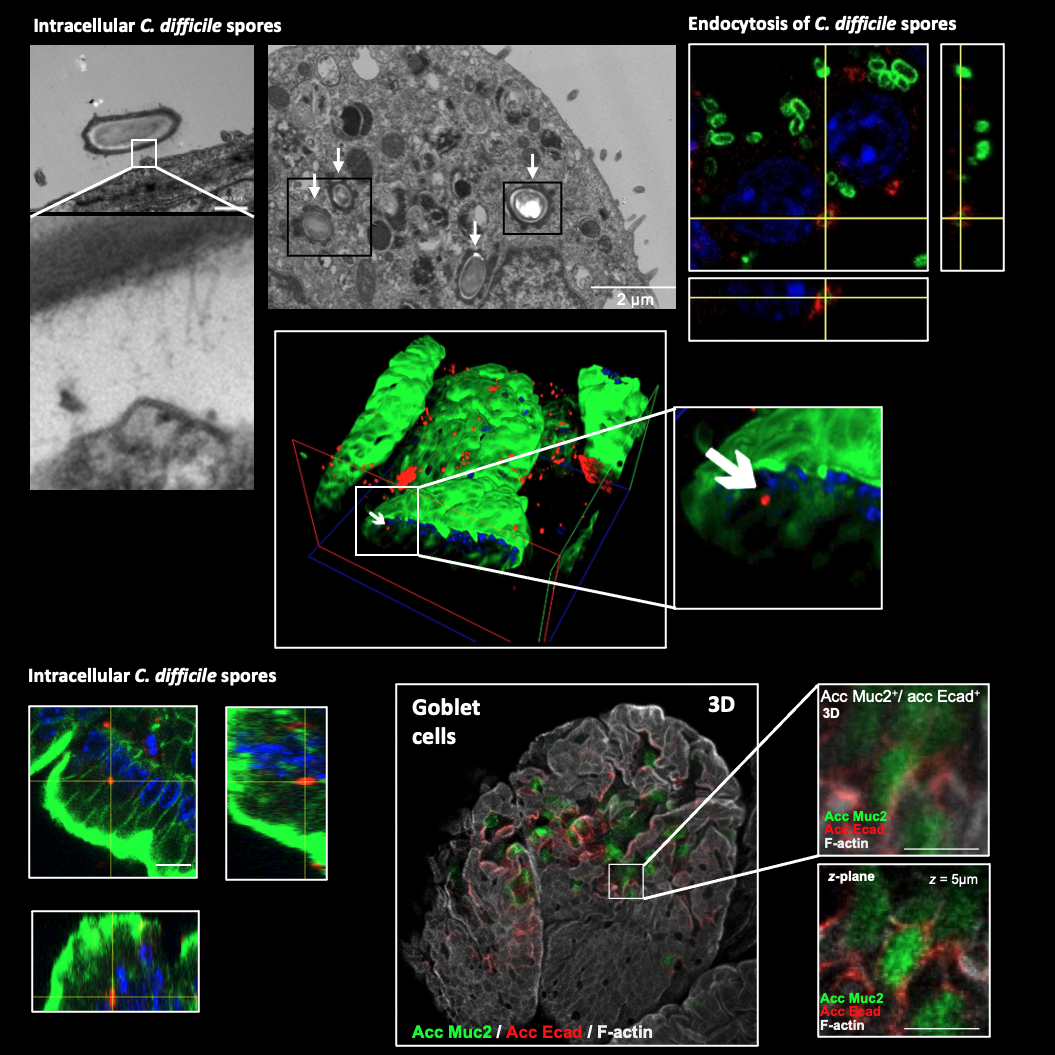
Some of our recent work shows that C. difficile spores gain entry into the intestinal mucosa via pathways dependent on host fibronectin-α5β1 and vitronectin-αvβ1. The exosporium protein BclA3, on the spore surface, is required for both entry pathways. Deletion of the bclA3 gene in C. difficile, or pharmacological inhibition of endocytosis using nystatin, leads to reduced entry into the intestinal mucosa and reduced recurrence of the disease in a mouse model. Our findings indicate that C. difficile spore entry into the intestinal barrier can contribute to spore persistence and infection recurrence, and suggest potential avenues for new therapies.
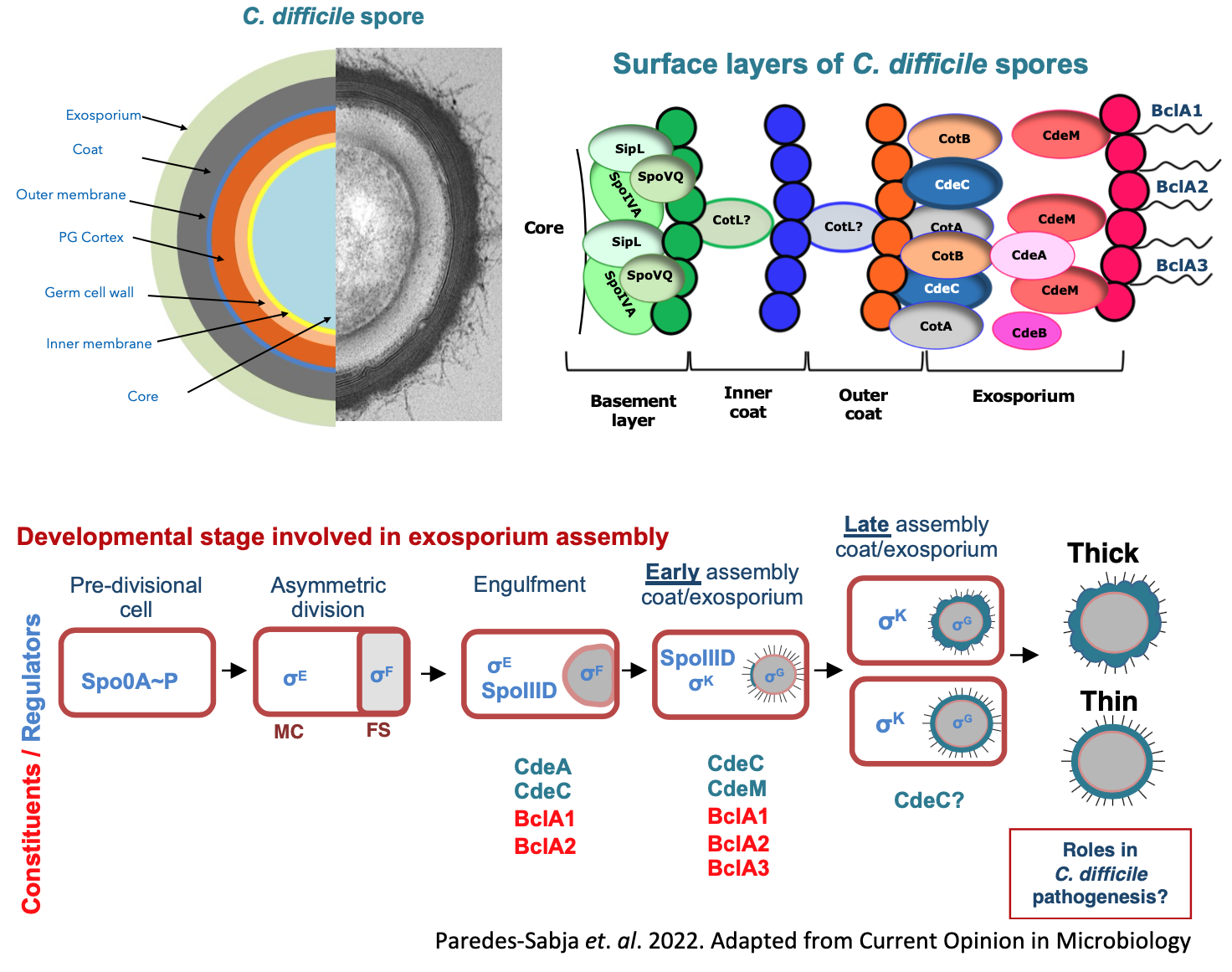
- Exosporium assembly mechanisms of C. difficile spores. The surface of C. difficile spores serves as the primary site of interaction with host surfaces. A major obstacle to develop therapies that remove C. difficile spores from the host and/or environment is the lack of basic understanding of how C. difficileforms the outer exosporium layer of the spore. Our aim is to understand how exosporium assembly occurs during C. difficilespore-formation. To dissect the basis underlying exosporium assembly we use molecular biology, bacterial genetics, biochemical and structural analysis, omics and single cell imaging techniques to understand the mechanisms through which key morphogenetic proteins (i.e., CdeA, CdeC and CdeM) drive exosporium assembly and interplay with other spore-coat and exosporium constituents (BclA1, BclA2 and BclA3).
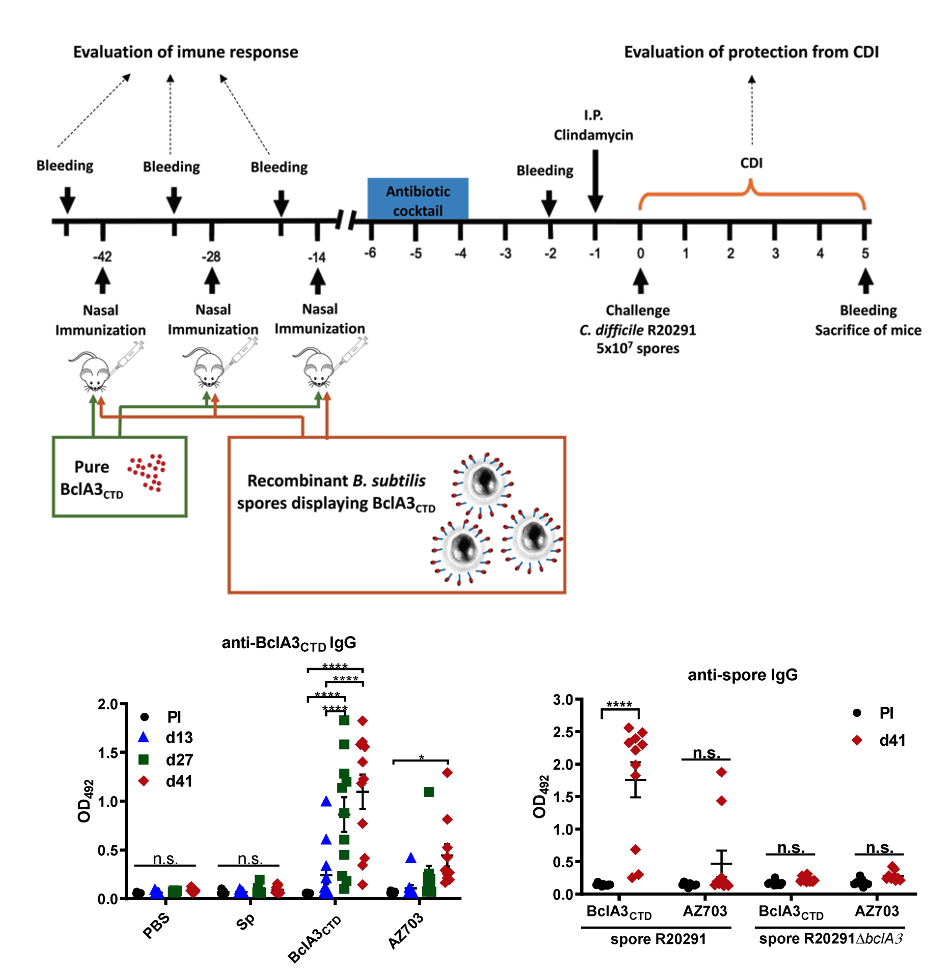
- Therapeutic development against C. difficile infections. Vaccines under development have only focused on the neutralization of TcdA and TcdB to generate systemic IgG anti-toxin immunity. Three vaccines have been tested in clinical trial: Sanofi Pasteur CDIFFENSE, (formalin-inactivated toxoid formulation); Valneva VLA84, (fusion toxin-protein); and Pfizer (non-toxigenic C. difficile strain, expressing non-infective recombinant Toxin A and Toxin B). Unfortunately, all three have been terminated their clinical trials due to failure to provide significant protective efficacy. No effective antibiotic and/or vaccine is readily available to resolve CDI and prevent recurrence and transmission of the disease. Consequently, derived from our understanding of how C. difficile interacts with the host and the composition of the spore surface layer, we aim to develop novel therapeutic strategies that prevent C. difficile spore´s interaction with the host´s intestinal mucosa and their persistence during disease. To accomplish these aims we blend knowledge from immune-proteomics and immunology, pharmacology and nanotechnology to design novel alternatives to combat CDI and prevent recurrence and transmission of the disease.
Texas A&M University I TAMU Biology Department I Dr. Daniel Paredes-Sabja Faculty profile I Dr. Marjorie Pizarro-Guajardo Profile
