Soil samples from sporotrichosis transmission belt area: Searching for fungal species and their antagonistic activity against Sporothrix brasiliensis
- 1Laboratory of Taxonomy, Biochemistry and Bioprospecting of Fungi, Oswaldo Cruz Institute, FIOCRUZ, Rio de Janeiro, Brazil
- 2Postdoctoral in Clinical Research in Infectious Diseases, Evandro Chagas National Institute of Infectious Diseases, FIOCRUZ, Rio de Janeiro, Brazil
- 3Laboratory of Clinical Research in Dermatozoonoses in Domestic Animals, Evandro Chagas National Institute of Infectious Diseases, FIOCRUZ, Rio de Janeiro, Brazil
Since 1998, the state of Rio de Janeiro, Brazil, has become a public health problem regarding sporotrichosis, a disease caused by Sporothrix spp. involving contact with infected cats. Efforts to isolate these species from environmental sources are not always successful. In our study, soil from residences situated in cities of Rio de Janeiro where cats with sporotrichosis live was collected and cultured an attempt to isolate Sporothrix spp. but it was not successful. However, other saprophytic fungal species were isolated from soil and identified and among them Purpureocillium lilacinum was the most frequent. From there, we decided to study the in vitro interaction of this species with S. brasiliensis, the principal agent that causes sporotrichosis in this state. The results showed that ten isolates of P. lilacinum inhibited the radial mycelial growth of S. brasiliensis with different percentage of inhibition. The interaction between them revealed the pattern described as overgrowth by antagonist. In conclusion, our data suggest that fungal species with very fast growth and capable of producing metabolites could hinder the growth of Sporothrix spp., it also opens the way for the identification of secondary metabolites with biological activity that could be tested against pathogenic fungi.
Introduction
Sporotrichosis is a subacute or chronic infection caused by the thermodimorphic species of the genus Sporothrix. It is a cosmopolitan disease of tropical and subtropical regions and is the most frequent subcutaneous mycosis in Latin America (Orofino-Costa et al., 2017).
Since 1998 this mycosis has become a public health problem in the state of Rio de Janeiro, Brazil, due to the significant increase in human and feline cases (Schubach et al., 2008; Gremião et al., 2020). In general, in Brazil, the transmission of this mycosis is by traumatic inoculation of fungi with the handling of organic matter. However, in the great metropolitan area of Rio de Janeiro was observed a zoonotic transmission from infected cats to humans (Orofino-Costa et al., 2017).
An exploratory analysis of the socio-spatial distribution of sporotrichosis in Rio de Janeiro state, from 1997 to 2007, identified a transmission belt along the border between the city of Rio de Janeiro and the adjacent municipalities in the greater metropolitan area (Silva et al., 2012). Recently, the epidemiological bulletin on sporotrichosis in this same state showed cases of sporotrichosis in municipalities in all administrative regions of RJ (Brasil, 2021). Molecular analysis clearly demonstrated that Sporothrix brasiliensis is the primary cause of zoonotic disease in Rio de Janeiro state (Oliveira et al., 2011; Gremião et al., 2020).
According to Poester et al. (2018) the environment can have a function as a reservoir for S. brasiliensis in hyperendemic areas of feline sporotrichosis, which may transfer the fungus from the sick animal to the environment by contact of animal lesions with surrounding; by feces of sick felines; or by burying the bodies of sick felines. However, environmental studies with isolation of Sporothrix species are not performed frequently and when done the results are not always successful. The reasons why there are difficulties in isolating Sporothrix species from soil are still not clear, but it is known that saprophytic species can have antagonistic actions on the growth of each other (Boddy and Hiscox, 2016).
The aims of this study were to evaluate the presence of Sporothrix spp. in soil samples from areas considered to be the transmission belt of sporotrichosis in the Rio de Janeiro and analyze antagonistic activities of saprophytic fungi against S. brasiliensis.
Materials and methods
Soil samples
The samples were collected from soil of residences, in which cats with sporotrichosis live, located in Atlantic Forest areas, in the cities of Petrópolis and Vassouras, metropolitan and central-south regions of Rio de Janeiro state, respectively. These areas are situated in the state’s sporotrichosis transmission belt (Silva et al., 2012; Brasil, 2021). Approximately 50g of soil were collected 10 cm below the topsoil and stored in Falcon tubes to be transported to the laboratory.
Isolation of fungi from soil samples
Fungi were isolated from soil using the serial dilution technique and inoculation in Petri dish containing potato dextrose agar medium (PDA – Difco, Becton-Dickinson and Company, New Jersey, EUA) with chloramphenicol (Adapted from Clark, 1965). Briefly, one gram of soil was weighed and placed in a 28 x 150 mm test tube. Sterile distilled water (10 mL) was added and the solution homogenized in vortex for 3 minutes. After, 1 mL of the soil solution was transferred to a test tube containing 9 mL of sterile distilled water, and so on to make serial dilutions (10-1 to 10-5). From the dilution 10-3 aliquots of 0.1 mL of solution was transferred to Petri dishes containing PDA (Difco) plus 0.05 g.mL-1 of chloramphenicol for inhibiting bacteria. They were placed in a BOD-type temperature chamber at 28°C ± 1°C, for seven days with daily monitoring.
The most representative colony forming units (CFU) were isolated and transferred to test tubes containing the same medium described above and kept in a BOD-type air-conditioned chamber with a controlled temperature of 28°C for future identification.
Identification by phenotypic characteristics of the fungal isolates
The analysis of macroscopic characteristics was made using the PDA medium and incubation at 28°C. The colonies were measured with a Mitutoyo digital caliper (Mitutoyo America Corporation, Aurora, Illinois, USA) with a resolution of 0.01mm. Some characteristics that are relevant for identification were observed, such as: texture, coloration of conidia, coloration of mycelium and reverse, presence and characterization of exudate, soluble pigments (Barron, 1972; Barnett and Hunter, 1998).
Microcultures (Riddell, 1950) using PDA medium for 7 days, at 28°C, were done for micromorphological evaluation and species identification. The morphology was observed after staining with Amann lactophenol plus cotton blue solution and examined under a Nikon light microscope, E400 model (Nikon Instruments Inc., Melville, NY, United States).
Screening by dual culture method
Antagonistic activity of the species isolated from soil was evaluated against S. brasiliensis type strain CBS120339 using dual culture assay (adapted from Liu et al., 2020). Using a platinum loop a constant quantity of fungi (the putative antagonist and S. brasiliensis) were separately point inoculated on PDA medium and placed 2 cm from the edge of the Petri dish at opposite ends. As a control, S. brasiliensis was placed in a similar manner on a fresh PDA plate. All pairings were carried out in triplicate and incubated at 28°C for seven days. Antagonistic activity was evaluated according to Rahman et al. (2009) by measuring the growth of both fungi in test and using the formula developed by Skidmore and Dickinson (1976): PIRG = R1 –R2/R1 x 100, where PIRG is percentage inhibition of radial growth; R1 is radius of the S. brasiliensis colony and R2 is radius of the putative antagonist colony. Interaction between species was monitored until the 28th day of incubation at same temperature.
Results
Fungal species from soil samples
The total number of 143 CFU was obtained from the soil samples per municipality, 46 from Petrópolis (Pe-46) and 97 from Vassouras (Va-97). Sporothrix sp. was not isolated from soil samples.
Of this total 137 colonies were filamentous fungi and 06 yeast-like colonies (not evaluated in the study). Eight genera were identified by classical taxonomy. Purpureocillium sp. presented the highest number of isolates 35 (Pe-6/Va-29), followed by Penicillium sp. with 16 (Pe-7/Va-9), Aspergillus sp. with 07 (Pe-1/Va-6), Beauveria sp. with 05 (Pe-5/Va-0), Trichoderma sp. with 05 (Pe-4/Va-1), Fusarium sp. with 05 (Pe-3/Va-2), Metarhizium sp. with 04 (Pe-3/Va-1) and Scopulariopsis sp. with 01 (Pe-1/Va-0) (Figure 1).
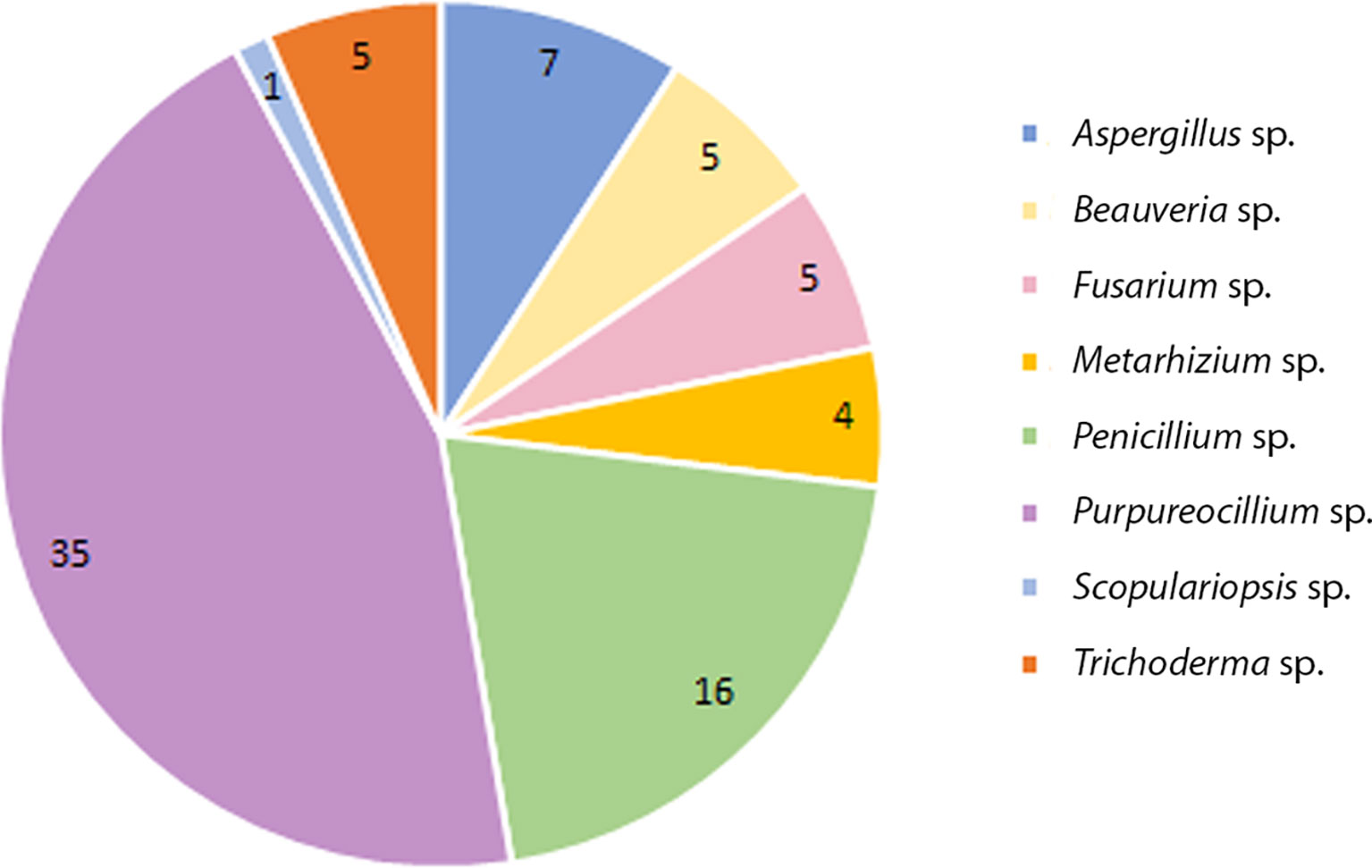
Figure 1 Total number of species collected from soil from domiciles with cases of feline sporotrichosis at Petrópolis and Vassouras, Rio de Janeiro state.
The species level identification of 63 isolates revealed: Purpureocillium lilacinum (Pe-6/Va-29); Penicillium citrinum (Pe-2/Va-4), Penicillium decumbens (Va-5), Penicillium expansum (Pe-2), Penicillium pinophilum (Pe-2), Penicillium oxalicum (Pe-1); Aspergillus versicolor (Pe-1/Va-3), Aspergillus fumigatus (Va-1), Aspergillus clavatus (Va-1), Aspergillus sydiwii (Va-1); Metharhizium anisoplae (3Pe-3/Va-1); Scopulariopsis brevicaulis (Pe-1). A group that did not produce any known spores was identified as Mycellia sterilia (4Pe, 21 Va).
Antagonistic activity of fungal species against Sporothrix brasiliensis
Three species found present in both municipalities (P. lilacinum; A. versicolor; M. anisopliae) were chosen for the screening tests. However, it was not possible to measure the inhibition potential of isolates of A. versicolor and M. anisopliae because their colonies overgrowth time was less than 7 days. On the other hand, ten isolates of P. lilacinum were tested and inhibited the radial mycelial growth of S. brasiliensis. The mean percentage inhibition of radial growth values (PIRG) ranged from 8% to 25% (Table 1).

Table 1 Inhibition (%) of growth of Sporothrix brasiliensis by antagonistic Purpureocillium lilacinum on potato dextrose agar at 7 days.
To illustrate the fungal antagonistic effect, the Figure 2 shows the growth inhibition of S. brasiliensis isolate by P. lilacinum isolates, LTBBF-PL4 (21.73%) and LTBBF-PL9 (18.18%), on the 7th day of incubation.

Figure 2 Antagonistic effects of Purpureocillium lilacinum isolates against Sporothrix brasiliensis in dual culture on the 7th day of incubation. Control plates on the left showing S. brasiliensis colony; dual culture plates on the right showing growth inhibition of S. brasiliensis by P. lilacinum: (A) LTBBF-PL4 (21.73% inhibition); (B) LTBBF-PL9 (18.18% inhibition).
The interaction between the two species revealed a pattern described by Porter (1924) apud Skidmore and Dickinson (1976) as overgrowth by antagonist (Figure 3).

Figure 3 Illustration of the interaction observed between two Purpureocillium lilacinum isolates (A LTBBF-PL4; B LTBBF-PL9) and Sporothrix brasiliensis on the 28th day of incubation. Control plates on the left show the growth of S. brasiliensis isolates and on the right plates show the interaction type “overgrowth by antagonist” which S. brasiliensis ceased its growth and was overgrown by P. lilacinum (arrows).
Discussion
In the last two decades, the state of Rio de Janeiro has become a hyperendemic area of cat-transmitted sporotrichosis, mainly caused by S. brasiliensis (Gremião et al., 2020). Although in Brazil the species with the highest occurrence is S. brasiliensis, its isolation from the environment is considered rare (Rabello et al., 2022).
Over the years, many efforts have already been made to isolate Sporothrix spp. from environmental sources in order to understand the ecology of these species, but it has not been an easy task resulting in low isolation rates compared to the amount of screening samples (Howard and Orr, 1963; Mackinnon et al., 1969; Kenyon et al., 1984; Dixon et al., 1991; Sanchez-Aleman et al., 2004; Mendoza et al., 2007; Metha et al., 2007; Criseo and Romeo, 2010; Cruz Choappa et al., 2014; Rodrigues et al., 2014) or unsuccessful isolations (Poester et al., 2018). Recently, Rabello et al. (2022) investigated the presence of Sporothrix spp. in samples of abandoned demolition woods collected from a house in Petrópolis, Rio de Janeiro, Brazil, which had a petcat with sporotrichosis. All efforts resulted in only one colony identified as S. brasiliensis isolated directly from the cat, not the environment in which it lived. Other environmental investigation collected soil samples from rural areas of two cities, Seropédica and Nova Iguaçu, Rio de Janeiro, Brazil, where zoonotic sporotrichosis is endemic (Almeida-Silva et al., 2022). However, no fungal growth compatible with Sporothrix spp. was observed by authors, but molecular techniques demonstrated the presence of DNA for S. brasiliensis in all samples. It is important to emphasize that the initial aim of this work was the attempt to isolate Sporothrix spp. from the soil of an endemic region, clarifying the gap of these studies mentioned above that, the same way as observed by us, were also unable to isolate the fungus. However, we recognize that the lack of use of molecular tools constitutes a deficiency of this study. Therefore, one of the future steps of our studies is to use these methodologies in order to refine our search for fungal species of interest.
In the present study we were also unsuccessful in isolating Sporothrix spp. from the soil, and the question is: what makes this isolation difficult? What kind of interaction in the soil could be hindering the growth of some species? Therefore, in an attempt to understand what would hinder the growth of Sporothrix spp., we decided to study in vitro the interaction of some P. lilacinum isolates (the species most isolated from the soil evaluated here and whose results were quantified) with one S. brasiliensis isolate. To understand how fungi, especially in the soil, interact, it is necessary to be aware that fungi grow in competition with other microorganisms and can overcome competition by rapid growth, sporulation, stress recovery, and the use and negation of inhibitors (Knowles et al., 2022). According to Almeida-Silva et al. (2022) some factors, among them, fungal faster growth rates, can limit the growth of pathogenic fungi, for example, Sporothrix spp. One of the strategies used by fungi in competition for habitat is the so-called antagonist effect. This effect is detected during mycelial interaction but can occur without mycelial contact, by the production of volatile and diffusible organic compounds (Boddy and Hiscox, 2016). In our experiment, was verified and quantified the inhibition in vitro of one S. brasiliensis isolate by ten P. lilacinum isolates showing different percentage of inhibition. Variation in ability to inhibit fungal isolates was also observed by Sonkar (2018) who described excellent antagonistic potentials differing between Trichoderma strains against Fusarium oxysporum. Isolates of Trichoderma antagonized Ceratocystis paradoxa growth and different strains also showed different degrees of inhibition (Rahman et al., 2009).
Previous studies point to the use of P. lilacinum as a biocontrol agent, since it is described as insect parasites (Barra et al., 2015; Hotaka et al., 2015) and nematodes (Singh et al., 2013; Liu et al., 2014). In addition, researchers have reported the ability of this species to inhibit the growth of other fungi, such as Penicillium digitatum (Elsherbiny et al., 2021) and Verticillium dahliae (Lan et al., 2017). But, how does this species interact, in vitro, with one of the agents of sporotrichosis? Our experiments demonstrated that the colony of S. brasiliensis ceased its growth and was overgrown by P. lilacinum, interaction classified as “overgrown antagonist” (Skidmore and Dickinson, 1976). Liu et al. (2020) noticed that P. lilacinum inhibited the growth of Botritys cinerea without overlapping or even physical contact between the two fungi, but they evaluated this interaction only by 7 days. On the 7th day, in our experiment, it was also observed that all P. lilacinum isolates did not overlap the S. brasiliensis colony (Figure 2), only doing so during the 28 days of incubation.
One of the possible mechanisms for antagonism between fungi is the production of organic compounds, so-called secondary metabolites that can alter spore germination, mycelial morphology, foraging behavior, and enzyme production (Boddy and Hiscox, 2016). Therefore, it is plausible to suppose that compounds produced by the P. lilacinum isolates tested here may have inhibited the growth of S. brasiliensis isolate. This hypothesis is supported by the study of Liu et al. (2020) who discovery a new antifungal lipopeptaibol (leucinostatin Z) from P. lilacinum against B. cinerea after co-culturing of two fungi on agar plate. In addition, P. lilacinum is reported as a species that produces different metabolites already characterized as leucinostatins, paecilotoxin and mycotoxin (Mikami et al., 1989; Khan et al., 2003; Park et al., 2004; Sharma and Sharma, 2016).
In summary, our results confirm the difficulty in isolating Sporothrix spp. from environmental sources and point out that very likely fungal species with very fast growth and capable of producing metabolites are the agents of this difficulty. Furthermore, this study paves the way for the isolation, purification and future identification of P. lilacinum secondary metabolites with biological activity that could be tested against pathogenic fungi.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
GC, AA and IF carried out experiments. AM wrote the original manuscript. DC-M analyzed the data. DC-M and CB wrote the manuscript. MO designed the study, acquired funding and administrated the project. DC-M, SP, CB and MO revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ - Grants: JCNE E-26/203.301/2017, JCNE E-26/201.433/2021– MO; E-26/202.737/2019 - SP), CAPES (DC-M fellowship 88882.317297/2019-01), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Grant Proc. 409227/2016-1 – MO; CNPq 312238/2020-7 - SP).
Acknowledgments
We are grateful to Fiocruz and all state funding agencies to support this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almeida-Silva, F., Rabello, V. B. S., Scramignon-Costa, B. S., Zancopé-Oliveira, R. M., de Macedo, P. M., Almeida-Paes, R. (2022). Beyond domestic cats: Environmental detection of Sporothrix brasiliensis DNA in a hyperendemic area of sporotrichosis in Rio de Janeiro state, Brazil. J. Fungi (Basel). 8 (6), 604. doi: 10.3390/jof8060604
Barnett, H. L., Hunter, B. B. (1998). Illustrated genera of imperfect fungi. 4th Edition (St. Paul: APS Press), 218.
Barra, P., Etcheverry, M., Nesci, A. (2015). Improvement of the insecticidal capacity of two Purpureocillium lilacinum strains against Tribolium confusum. Insects 6 (1), 206–223. doi: 10.3390/insects6010206
Barron, G. L. (1972). The genera of hyphomycetes from soil (New York: Robert E. Krieger Publishing Company), 364.
Boddy, L., Hiscox, J. (2016). Fungal ecology: Principles and mechanisms of colonization and competition by saprotrophic fungi. Microbiol. Spectr. 4 (6). doi: 10.1128/microbiolspec.FUNK-0019-2016
Brasil (2021). “Secretaria do estado de saúde do Rio de Janeiro – GERDTVZ boletim epidemiológico esporotricose,” in Cenário epidemiológico da esporotricose no estado do Rio de Janeiro – anos de 2019 e 2020.
Clark, F. E. (1965). Agar-platemethod for total microbial count. in methods of soil analysis, part 2. chemicaland microbiological properties. Eds. Blanc, C. A., Evans, D., White, J. L., Ensminger, L. E., Clark, F. E., Dinauer, R. C. (New York: Madson Inc.), 1460–1466.
Criseo, G., Romeo, O. (2010). Ribosomal DNA sequencing and phylogenetic analysis of environmental Sporothrix schenckii strains: comparison with clinical isolates. Mycopathologia 169 (5), 351–358. doi: 10.1007/s11046-010-9274-9
Cruz Choappa, R. M., Vieille Oyarzo, P. I., Carvajal Silva, L. C. (2014). Isolation of Sporothrix pallida complex in clinical and environmental samples from Chile. Rev. Argent Microbiol. 46 (4), 311–314. doi: 10.1016/S0325-7541(14)70088-4
Dixon, D. M., Salkin, I. F., Duncan, R. A., et al. (1991). Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J. Clin. Microbiol. 29 (6), 1106–1113. doi: 10.1128/jcm.29.6.1106-1113.1991
Elsherbiny, E. A., Taher, M. A., Abd El-Aziz, M. H., Mohamed, S. Y. (2021). Action mechanisms and biocontrol of Purpureocillium lilacinum against green mould caused by Penicillium digitatum in orange fruit. J. Appl. Microbiol. 31 (3), 1378–1390. doi: 10.1111/jam.15016
Gremião, I. D. F., Oliveira, M. M. E., Monteiro de Miranda, L. H., Saraiva Freitas, D. F., Pereira, S. A. (2020). Geographic expansion of sporotrichosis, Brazil. Emerg. Infect. Dis. 26 (3), 621–624. doi: 10.3201/eid2603.190803
Hotaka, D., Amnuaykanjanasin, A., Chan, M., Siritutsoontorn, S., Maketon, M. (2015). Efficacy of Purpureocillium lilacinum CKPL-053 in controlling Thrips palmi (Thysanoptera: Thripidae) in orchid farms in Thailand. App. Entomol Zool. 58, 1–13.
Howard, D. H., Orr, G. F. (1963). Comparison of strains of Sporotrichum schenckii isolated from nature. J. Bacteriol. 85 (4), 816–821. doi: 10.1128/Jb.85.4.816-821.1963
Kenyon, E. M., Russell, L. H., McMurray, D. N. (1984). Isolation of Sporothrix schenckii from potting soil. Mycopathologia 87 (1-2), 128. doi: 10.1007/BF00436641
Khan, A., Williams, K., Nevalainen, H. (2003). Testing the nematophagous biological control strain paecilomyces lilacinus 251 for paecilotoxin production. FEMS Microbiol. Lett. 227 (1), 107–111. doi: 10.1016/S0378-1097(03)00654-2
Knowles, S. L., Raja, H. A., Roberts, C. D., Oberlies, N. H. (2022). Fungal-fungal co-culture: a primer for generating chemical diversity. Nat. Prod. Rep. 10. doi: 10.1039/d1np00070
Lan, X., Zhang, J., Zong, Z., Ma, Q., Wang, Y. (2017). Evaluation of the biocontrol potential of Purpureocillium lilacinum QLP12 against Verticillium dahliae in eggplant. BioMed. Res. Int. 2017, 4101357. doi: 10.1155/2017/4101357
Liu, R., Khan, R. A. A., Yue, Q., et al. (2020). Discovery of a new antifungal lipopeptaibol from Purpureocillium lilacinum using MALDI-TOF-IMS. Biochem. Biophys. Res. Commun. 527 (3), 689–695. doi: 10.1016/j.bbrc.2020.05.021
Liu, J., Sun, J., Qiu, J., Liu, X., Xiang, M. (2014). Integrated management of root-knot nematodes on tomato in glasshouse production using nematicides and a biocontrol agent, and their effect on soil microbial communities. Nematology 16 (4), 463–473. doi: 10.1163/15685411-00002778
Mackinnon, J. E., Conti-Díaz, I. A., Gezuele, E., Civila, E., da Luz, S. (1969). Isolation of Sporothrix schenckii from nature and considerations on its pathogenicity and ecology. Sabouraudia 7 (1), 38–45.
Mehta, K. I., Sharma, N. L., Kanga, A. K., Mahajan, V. K., Ranjan, N. (2007). Isolation of Sporothrix schenckii from the environmental sources of cutaneous sporotrichosis patients in himachal pradesh, India: results of a pilot study. Mycoses 50 (6), 496–501. doi: 10.1111/j.1439-0507.2007.01411.x
Mendoza, M., Diaz, E., Alvarado, P., Romero, E., Bastardo de Albornoz, M. C. (2007). Isolation of Sporothrix schenckii from environmental samples in Venezuela. Rev. Iberoam Micol. 24 (4), 317–319. doi: 10.1016/s1130-1406(07)70064-5
Mikami, Y., Yazawa, K., Fukushima, K., Arai, T., Udagawa, S., Samson, R. A. (1989). Paecilotoxin production in clinical or terrestrial isolates of Paecilomyces lilacinus strains. Mycopathologia 108 (3), 195–199. doi: 10.1007/BF00436225
Oliveira, M. M. E., Almeida-Paes, R., Muniz, M. M., Gutierrez-Galhardo, M. C., Zancope-Oliveira, R. M. (2011). Phenotypic and molecular identification of sporothrix isolates from an epidemic area of sporotrichosis in Brazil. Mycopathologia 172 (4), 257–267. doi: 10.1007/s11046-011-9437-3
Orofino-Costa, R., Macedo, P. M., Rodrigues, A. M., Bernardes-Engemann, A. R. (2017). Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. Bras. Dermatol. 92 (5), 606–620. doi: 10.1590/abd1806-4841.2017279
Park, J. O., Hargreaves, J. R., McConville, E. J., Stirling, G. R., Ghisalberti, E. L., Sivasithamparam, K. (2004). Production of leucinostatins and nematicidal activity of Australian isolates of Paecilomy.ces lilacinus (Thom) Samson. Lett. Appl. Microbiol. 38 (4), 271–276. doi: 10.1111/j.1472-765x.2004.01488.x
Poester, V.R., Mendes, J.F., Von Groll, A., Baracy Klafke, G., Brandolt, T.M., Xavier, M.O. (2018). Avaliação da presença de sporothrix spp. em solo de área hiperendêmica para esporotricose no extremo sul do brasil. Braz. Anim. Sci. 19, 1–8.
Porter, C. L. (1924). Concerning the characters of certain fungi as exhibited by their growth in the presence of other fungi. Am. J. Bo. 11, 168–188.
Rabello, V. B. S., Almeida-Silva, F., Scramignon-Costa, B. S., et al. (2022). Environmental isolation of Sporothrix brasiliensis in an area with recurrent feline sporotrichosis cases. Front. Cell Infect. Microbiol. 2. doi: 10.3389/fcimb.2022.894297
Rahman, M. A., Begum, M. F., Alam, M. F. (2009). Screening of Trichoderma isolates as a biological control agent against Ceratocystis paradoxa causing pineapple disease of sugarcane. Mycobiology 37 (4), 277–285. doi: 10.4489/MYCO.2009.37.4.277
Riddell, R. W. (1950). Permanent stained mycological preparations obtained by slide culture. Mycologia 42 (2), 265–270. doi: 10.2307/3755439
Rodrigues, A. M., Bagagli, E., de Camargo, Z. P., Bosco Sde, M. (2014). Sporothrix schenckii sensu stricto isolated from soil in an armadillo's burrow. Mycopathologia 177 (3-4), 199–206. doi: 10.1007/s11046-014-9734-8
Sánchez-Alemán, M. A., Araiza, J., Bonifaz, A. (2004). Aislamiento y caracterización de cepas silvestres de Sporothrix schenckii e investigación de reactores a la esporototicina. Gac Méd Méx. 140 (5), 507–512.
Schubach, A., Barros, M. B., Wanke, B. (2008). Epidemic sporotrichosis. Curr. Opin. Infect. Dis. 21 (2), 129–133. doi: 10.1097/QCO.0b013e3282f44c52
Sharma, A., Sharma, S. (2016). Production of secondary nematicidal metabolites from Purpureocillium lilacinum using karanja deoiled cake. Int. J. Trop. Agric. 34, 733–737.
Silva, M. B., Costa, M. M., Torres, C. C., et al. (2012). Esporotricose urbana: epidemia negligenciada no Rio de Janeiro, brasil [Urban sporotrichosis: a neglected epidemic in Rio de Janeiro, Brazil. Cad. Saude Publica. 28 (10), 1867–1880. doi: 10.1590/s0102-311x2012001000006
Singh, S., Pandey, R. K., Goswami, B. K. (2013). Bio-control activity of Purpureocillium lilacinum strains in managing root-knot disease of tomato caused by Meloidogyne incognita. Bioc. Sci. Technol. 23 (12), 1469–1489. doi: 10.1080/09583157.2013.840770
Skidmore, A. M., Dickinson, C. H. (1976). Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans. Brit. Mycol. Soc 66, 57–64.
Keywords: environment, soil, fungal interaction, phenotypic characterization, Sporothrix spp., sporotrichosis, Purpureocillium lilacinum
Citation: Lara da Costa G, Escórcio Ferreira I, Corrêa-Moreira D, Marinho A, Benedito de Almeida A, Antônio Pereira S, Moraes Borba C and Marques Evangelista Oliveira M (2022) Soil samples from sporotrichosis transmission belt area: Searching for fungal species and their antagonistic activity against Sporothrix brasiliensis. Front. Cell. Infect. Microbiol. 12:1033969. doi: 10.3389/fcimb.2022.1033969
Received: 01 September 2022; Accepted: 07 November 2022;
Published: 01 December 2022.
Edited by:
Domenico Giosa, University of Messina, ItalyReviewed by:
Luana P. Borba-Santos, Federal University of Rio de Janeiro, BrazilEmeka Nweze, University of Nigeria, Nsukka, Nigeria
Copyright © 2022 Lara da Costa, Escórcio Ferreira, Corrêa-Moreira, Marinho, Benedito de Almeida, Antônio Pereira, Moraes Borba and Marques Evangelista Oliveira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manoel Marques Evangelista Oliveira, manoel.marques@ioc.fiocruz.br; Danielly Corrêa-Moreira, dcorrea@ioc.fiocruz.br
 Gisela Lara da Costa
Gisela Lara da Costa Isabella Escórcio Ferreira1
Isabella Escórcio Ferreira1