An enigmatic beetle with affinity to Lamingtoniidae in mid-Cretaceous amber from northern Myanmar (Coleoptera: Cucujoidea)
- 1State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China
- 2Center for Excellence in Life and Paleoenvironment, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China
- 3School of Earth Sciences, University of Bristol, Bristol, United Kingdom
- 4Manaaki Whenua Landcare Research, New Zealand Arthropod Collection, Auckland, New Zealand
- 5Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Laboratory of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, Guangzhou, China
An enigmatic cucujiform beetle, Alloterocucus atratus Li, Leschen, Liu, and Cai gen. et sp. nov., is reported from mid-Cretaceous Burmese amber. The character combination of the new fossil is not completely consistent with any of the known cucujoid or erotyloid families. Based on our phylogenetic analyses, Alloterocucus is assigned to Cucujoidea and may be allied to Lamingtoniidae, which contains a single Australasian genus in the extant fauna. Alloterocucus shares with Lamingtoniidae a similar habitus and a series of characters, including the absence of postocular constriction, 3-segmented antennal club, externally open procoxal cavities, laterally open mesocoxal cavities, exposed pro- and mesotrochantins, and the absence of epipleural fovea and pronotal setose cavities, but differs from extant Lamingtoniidae in its apically truncate terminal maxillary palpomeres, 5-5-4 tarsi in male and absence of distinct dorsal punctation.
Zoobank registration: [https://zoobank.org/], identifier [111CE15E-5B49-4154-9E4A-7B3A738C6D2A].
Introduction
The beetle superfamily Cucujoidea of the series Cucujiformia has a complex taxonomic history. Historically, it is essentially a group of families without clear diagnostic characteristics of other superfamilies (especially Tenebrionoidea; Crowson, 1955; Lawrence and Newton, 1982). The coccinelloid group, once regarded as the cerylonid series, was recognized based on multiple lines of morphological (Crowson, 1955; Ślipiński and Pakaluk, 1991) and molecular evidence (Hunt et al., 2007; Robertson et al., 2008, 2015; Bocak et al., 2014), and formally removed from Cucujoidea and elevated to its superfamilial status by Robertson et al. (2015). The phylogenetic relationships within the remaining Cucujoidea vary dramatically among various morphological and molecular studies (e.g., Leschen et al., 2005; Robertson et al., 2008, 2015; Lawrence et al., 2011; McElrath et al., 2015; Timmermans et al., 2016; Zhang et al., 2018; McKenna et al., 2019). Although some molecular analyses either based on a few gene markers (Robertson et al., 2015) or a larger dataset (95 nuclear protein-coding genes) (Zhang et al., 2018) under a site-homogeneous substitution model supported a monophyletic Cucujoidea sensu Robertson et al. (2015), recent studies using transcriptomic data (McKenna et al., 2019) or a better-fitting site-heterogeneous model (Cai et al., 2022) have consistently demonstrated the paraphyly of Cucujoidea sensu Robertson et al. (2015). In light of the more recent phylogenomic results, Cai et al. (2022) therefore formally divided Cucujoidea into three superfamilies, namely, Erotyloidea, Nitiduloidea, and Cucujoidea sensu stricto.
While some families can be characterized based on distinctive synapomorphies like Cyclaxyridae (Gimmel et al., 2019), parallelism and convergence of morphological characters in Cucujoidea sensu lato make it difficult to assign unusual beetles into an appropriate family, and this is no truer than amber fossils where the critical assessment of more subtle characters is required. Moreover, some of the more critical characters used for distinguishing Cucujoidea sensu stricto and Erotyloidea are internal genitalic characters (Cai et al., 2022; Gimmel and Leschen, 2022). For example, Pleuroceratos Poinar and Kirejtshuk from mid-Cretaceous Burmese amber was originally placed in Silvanidae, but this placement was later rejected by Liu et al. (2019) primarily based on its oblique procoxae with exposed protrochantins. Kirejtshuk et al. (2019) subsequently transferred Pleuroceratos to Sphindidae. However, Tihelka et al. (2020) suggested that Pleuroceratos is a member of Phloeostichidae based on a phylogenetic analysis of morphological characters.
Like the taxonomic case for the placement of Pleuroceratos, we have discovered an enigmatic Burmese amber fossil that requires a critical assessment of characters necessary for familial placement. This new taxon has a dorsal habitus similar to modern-day Erotylidae and Lamingtoniidae (Figure 1), though, unlike these families that have 5-5-5 tarsomeres in both sexes, this fossil has 5-5-4 tarsomeres, which is diagnostic for males of several families of Cucujoidea sensu stricto (Lawrence and Ślipiński, 2013). The position of the fossil is therefore subjected to phylogenetic analyses, and the results are discussed in the context of the current classification of Cucujoidea.

Figure 1. General habitus of Alloterocucus atratus Li, Leschen Liu, and Cai gen. et sp. nov., holotype, NIGP180056, under incident light. (A) Dorsal view. (B) Ventral view. Scale bars: 500 μm.
Materials and methods
Materials
The Burmese amber specimen studied herein (Figures 1–3) originated from amber mines near Noije Bum (26°20′ N, 96°36′ E), Hukawng Valley, Kachin State, northern Myanmar. The specimen is deposited in the Nanjing Institute of Geology and Palaeontology (NIGP), Chinese Academy of Sciences, Nanjing, China. The amber piece was trimmed with a small table saw, ground with emery papers of different grit sizes, and finally polished with polishing powder.
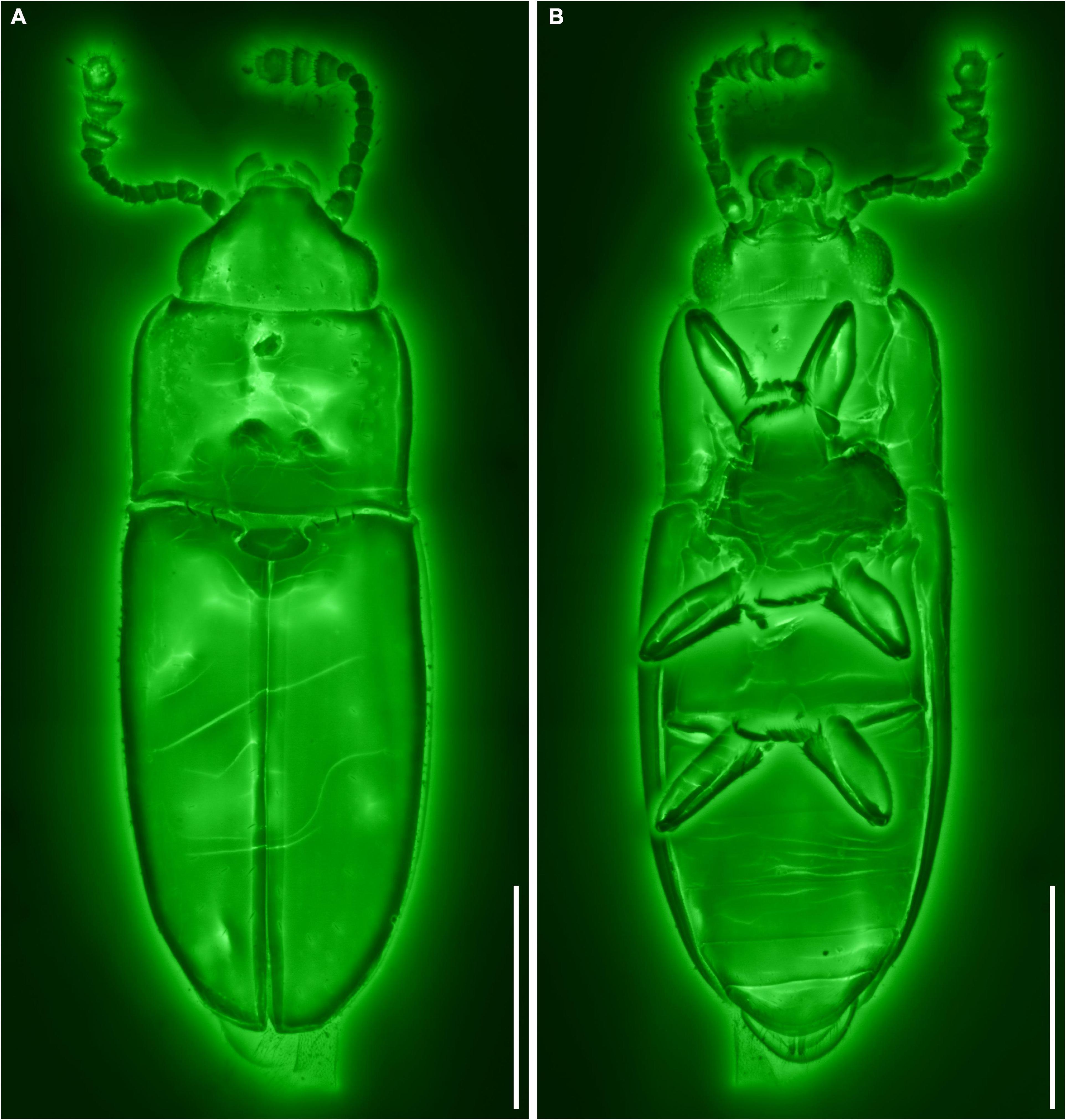
Figure 2. General habitus of Alloterocucus atratus Li, Leschen Liu, and Cai gen. et sp. nov., holotype, NIGP180056, under confocal microscopy. (A) Dorsal view. (B) Ventral view. Scale bars: 400 μm.
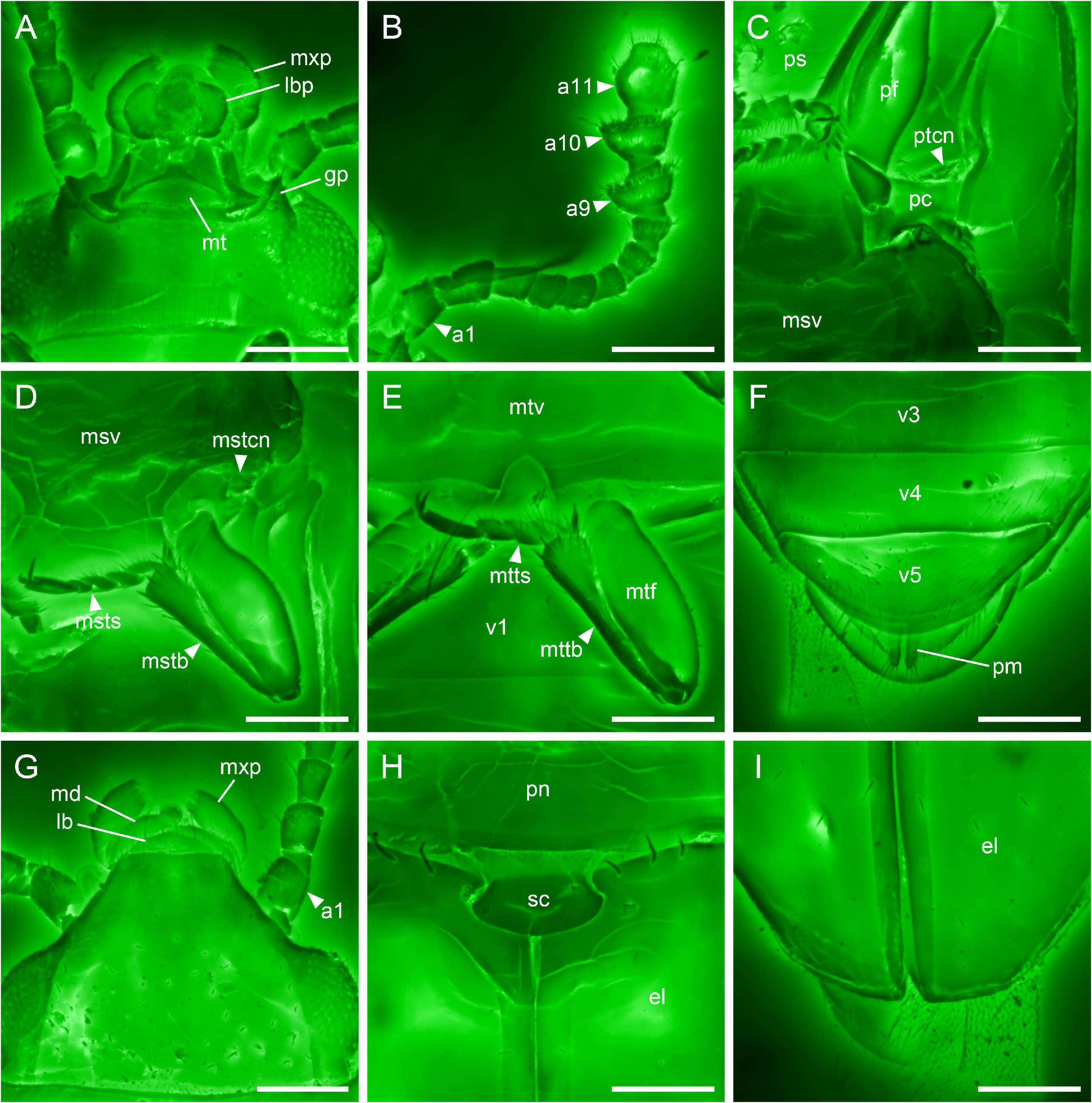
Figure 3. Details of Alloterocucus atratus Li, Leschen Liu, and Cai gen. et sp. nov., holotype, NIGP180056, under confocal microscopy. (A) Head, ventral view. (B) Antenna, ventral view. (C) Prothorax, ventral view. (D) Mid leg, ventral view. (E) Hind leg, ventral view. (F) Abdominal apex, ventral view. (G) Head, dorsal view. (H) Scutellum, dorsal view. (I) Elytral apex, dorsal view. a1–11, antennomeres 1–11; el, elytron; gp, genal projection; lb, labrum; lbp, labial palp; md, mandible; mstb, mesotibia; mstcn, mesotrochantin; msts, mesotarsus; msv, mesoventrite; mt, mentum; mtf, metafemur; mttb, metatibia; mtts, metatarsus; mtv, metaventrite; mxp, maxillary palp; pc, procoxa, pf, profemur; pm, paramere; pn, pronotum; ps, prosternum; ptcn, protrochantin; sc, scutellum; v1–5, ventrites 1–5. Scale bars: 100 μm.
Fossil imaging
Brightfield images were taken using a Zeiss Discovery V20 stereo microscope. Confocal images were obtained with a Zeiss LSM710 confocal laser scanning microscope, using the 488 nm Argon laser excitation line (Fu et al., 2021). Brightfield images were stacked in Helicon Focus 7.0.2 and Adobe Photoshop CC. Confocal images were stacked with color coding for depth in ZEN 3.4 (Blue Edition), or semi-manually stacked in Helicon Focus 7.0.2 and Adobe Photoshop CC. Images were further processed in Adobe Photoshop CC to adjust brightness and contrast.
Morphological phylogenetic analysis
To evaluate the systematic placement of the new species within Cucujoidea, we conducted formal morphological phylogenetic analyses under weighted parsimony. The data matrix (Supplementary Data 1, 2) was mainly derived from the previously published dataset by Leschen et al. (2005), which has the advantage of having been developed for Cucujoidea sensu lato prior to the classification in Cai et al. (2022). Therefore, we can determine if the new species is a member of Cucujoidea sensu stricto, Erotyloidea, or Nitiduloidea with outgroups from Cleroidea and Derodontoidea. The full matrix includes 66 adult and 33 larval characters, among which we successfully coded 36 adult characters for the new fossil.
Both parsimony analyses were performed under implied weights with R 4.1.0 (R Core Team, 2021) and the R package TreeSearch 1.0.1 (Smith, 2021; Supplementary Data 3). Parsimony analyses achieve the highest accuracy under a moderate weighting scheme (i.e., when concavity constants, K, are between 5 and 20) (Goloboff et al., 2018; Smith, 2019). Therefore, the concavity constant was set to 12 here, as suggested by Goloboff et al. (2018). In the unconstrained analysis, the clade support was generated based on 1,000 jackknife pseudoreplicates.
Since the morphology-based phylogeny of Cucujoidea was largely discordant with the molecular phylogeny, a constrained analysis was conducted as well (e.g., Slater, 2013; Fikáček et al., 2020). The interrelationships among extant families were fixed according to the synthesized tree (their figure 2) by McKenna et al. (2019). The intrafamilial relationships within extant Phloeostichidae and Priasilphidae were somewhat arbitrarily decided (partly based on Leschen et al., 2005). The fossil was allowed to move freely across the reference tree. An exhaustive search was conducted to find the best placement for the new genus.
The trees were drawn with the online tool iTOL 6.5.2 (Letunic and Bork, 2021) and graphically edited with Adobe Illustrator CC 2017.
Systematic paleontology
Order Coleoptera Linnaeus, 1758
Suborder Polyphaga Emery, 1886
Series Cucujiformia Lameere, 1938
Superfamily Cucujoidea Latreille, 1802
Family incertae sedis
Genus Alloterocucus Li, Leschen, Liu, and Cai gen. nov.
Type species
Alloterocucus atratus sp. nov.
Etymology
The generic name is formed based on the Greek “allótrios,” strange, and “cucujoid,” referring to its unique character combination within the traditional Cucujoidea. The name is masculine in gender.
Diagnosis
Body elongate. Postocular constriction absent (Figures 3A,G). Antennal club 3-segmented (Figure 3B). Apical maxillary palpomere subcylindrical, apically truncate (Figure 3A). Genal projections acute (Figure 3B). Ventral surface of head smooth, without any grooves (Figure 3B). Procoxal cavities externally open (Figure 3C). Mesocoxal cavities laterally open (Figure 3D). Pro- and mesotrochantins exposed (Figures 3C,D). Mesoventrite distinctly longer than half-length of metaventrite. Metaventral discrimen absent (Figure 3E). Elytra without well-developed punctures (Figures 3H,I). Tarsi 5-5-4 (probably in male) (Figures 3C–E).
Alloterocucus atratus Li, Leschen, Liu, and Cai sp. nov.
Material
Holotype, NIGP180056 (Figures 1–3), probably male (refer to Remarks), mid-Cretaceous (upper Albian to lower Cenomanian; Shi et al., 2012; Mao et al., 2018), from the amber mine near Noije Bum Village, Tanai Township, Myitkyina District, Kachin State, Myanmar.
Etymology
The specific name refers to the blackened (carbonized) appearance of the holotype.
Diagnosis
As for the genus.
Description
Body small, elongate, about 1.62 mm long, 0.56 mm wide.
Head prognathous, not constricted posteriorly, somewhat flattened; supra-antennal ridges or bead absent. Eyes moderately large, somewhat protuberant, entire, finely facetted, without interfacetal setae. Frontoclypeal suture absent; frontal carina on each side gradually narrowed anteriorly, almost reaching anterior end of clypeus; clypeus truncate anteriorly. Antennal insertions located laterally, hidden in dorsal view. Antennal grooves absent. Antennae 11-segmented, with distinct, 3-segmented club; antennomeres 9 and 10 transverse, slightly asymmetrical, with distinct rims. Labrum transverse, with broadly rounded apex. Maxillary palps with terminal palpomere relatively wide and somewhat cylindrical, not narrowed or dilated apically. Mentum basally with a transverse depression; deep or shallow lines extending backward from maxillary base absent. Genae produced anteriorly and acute. Ventral surface of head smooth, without any clear ridges or grooves.
Pronotum weakly transverse, about 1.4× as wide as long; lateral sides subparallel, with raised border; anterior angles slightly produced forward; posterior angles more or less right; posterior edge slightly bisinuate; disc lacking impressions. Prosternum in front of coxae longer than procoxal cavity; prosternal process relatively broad, laterally margined, with broadly rounded apex. Procoxal cavities transverse, without trochantinal notch, externally probably broadly open, with narrow lateral extensions. Procoxae not projecting. Protrochantins exposed.
Scutellar shield transverse, posteriorly broadly rounded. Elytra about 1.7 times as long as combined width and about 2.5 times as long as pronotum; width at base about as wide as width of pronotum; disc with scattered setae, without well-developed punctures; sutural striae present and complete from base to apex; epipleura gradually narrowed posteriorly, distinct toward apex; subapical gape absent. Mesoventrite relatively long and broad, about as long as half maximum width. Mesocoxal cavities moderately widely separated, open laterally, not closed by the meeting of mesoventrite and metaventrite. Mesotrochantins exposed. Mesometaventral junction slightly curved posteriorly, with metaventrite and mesoventrite coplanar. Metaventrite broad, without median discrimen. Metacoxae strongly transverse, narrowly separated; plates absent.
Legs relatively slender. Trochanterofemoral joints not strongly oblique, with femora not contacting coxae. Tibiae not parallel-sided and expanded toward apex with several stout setae along distal end; tibial spurs 2-2-2. Tarsi 5-5-4; tarsomeres not lobed ventrally; basal tarsomeres short and subequal; apical tarsomere longer. Pretarsal claws simple.
Abdomen with five ventrites; ventrite 1 not much longer than 2; intercoxal process narrowly rounded apically.
Remarks
A pair of segments bearing setae protruding from the abdominal apex are visible in the holotype, which might be interpreted as the styli of the female ovipositor. However, the 5-5-4 tarsi in females rarely occur outside of Tenebrionoidea, and other characters of Alloterocucus do not fit well with Tenebrionoidea. For example, Alloterocucus does not have the heteromeroid type trochanterofemoral joint (not strongly oblique) nor does it have projecting procoxae, externally closed procoxal cavities, or well-exposed protrochantins present in some similar groups of tenebrionoids. Alternatively, the protruding segments may be interpreted as the elongate parameres of a male specimen, similar to what may be present in Erotylidae. Many Cucujoidea sensu lato have dimorphic tarsal formulae with males having 5-5-4 and females with 5-5-5, and it is plausible that our Alloterocucus specimen is a male.
Discussion
The character combination of Alloterocucus is not completely accordant with any present-day cucujoid or erotyloid families. Our constrained phylogenetic analysis (Figure 4) excludes Alloterocucus from Erotyloidea, despite having a similar habitus to some dacnine Erotylidae. Furthermore, we can exclude Alloterocucus from Erotyloidea by the presence of 5-5-4 tarsomeres, and lack of visible glandular ducts and open mesocoxal cavities, which exclude it from Erotylidae (see below), and the absence of the frontoclypeal suture, which excludes it from Boganiidae. It can also be excluded from Cryptophagidae by the open mesocoxal cavities, the subequal basal abdominal ventrites, and the elytra with complete (albeit narrow) epipluera and no subapical gape. Alloterocucus is placed within the Cucujoidea sensu stricto in a trio consisting of Tasmosalpingidae, Cyclaxyridae, and Lamingtoniidae, as sister to Lamingtoniidae.
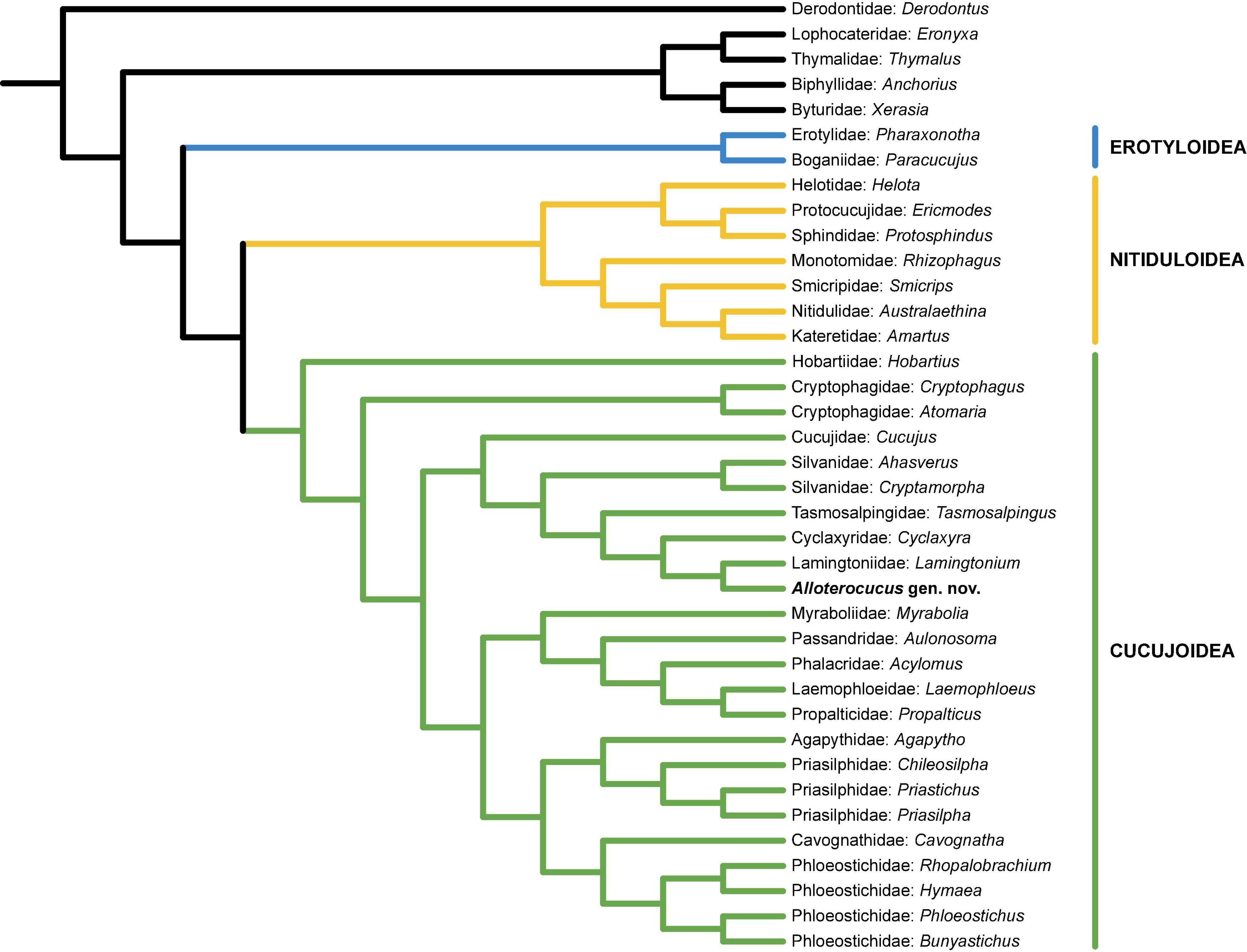
Figure 4. Placement of Alloterocucus Li, Leschen Liu, and Cai gen. nov. based on the single tree resulting from the constrained parsimony analysis.
In the unconstrained analysis (Figure 5), Alloterocucus was also grouped together with the same trio, but as sister to Cyclaxyridae. Therefore, we conclude that Alloterocucus is likely a member of Cucujoidea sensu stricto and related to these monogeneric Australasian families, which are absent from the fossil record, apart from Cyclaxyridae known from Burmese and Baltic ambers (Gimmel et al., 2019). Alloterocucus shares the following characters with these families: absence of postocular constriction, 3-segmented antennal club, externally open procoxal cavities, laterally open mesocoxal cavities, exposed pro- and mesotrochantins, and the absence of metaventral discrimen. Tasmosalpingidae and Cyclaxyridae additionally have 5-5-4 tarsi in males, and if we assume that the specimen of Alloterocucus is a male, this will support the classification of this genus in Cucujoidea sensu stricto.
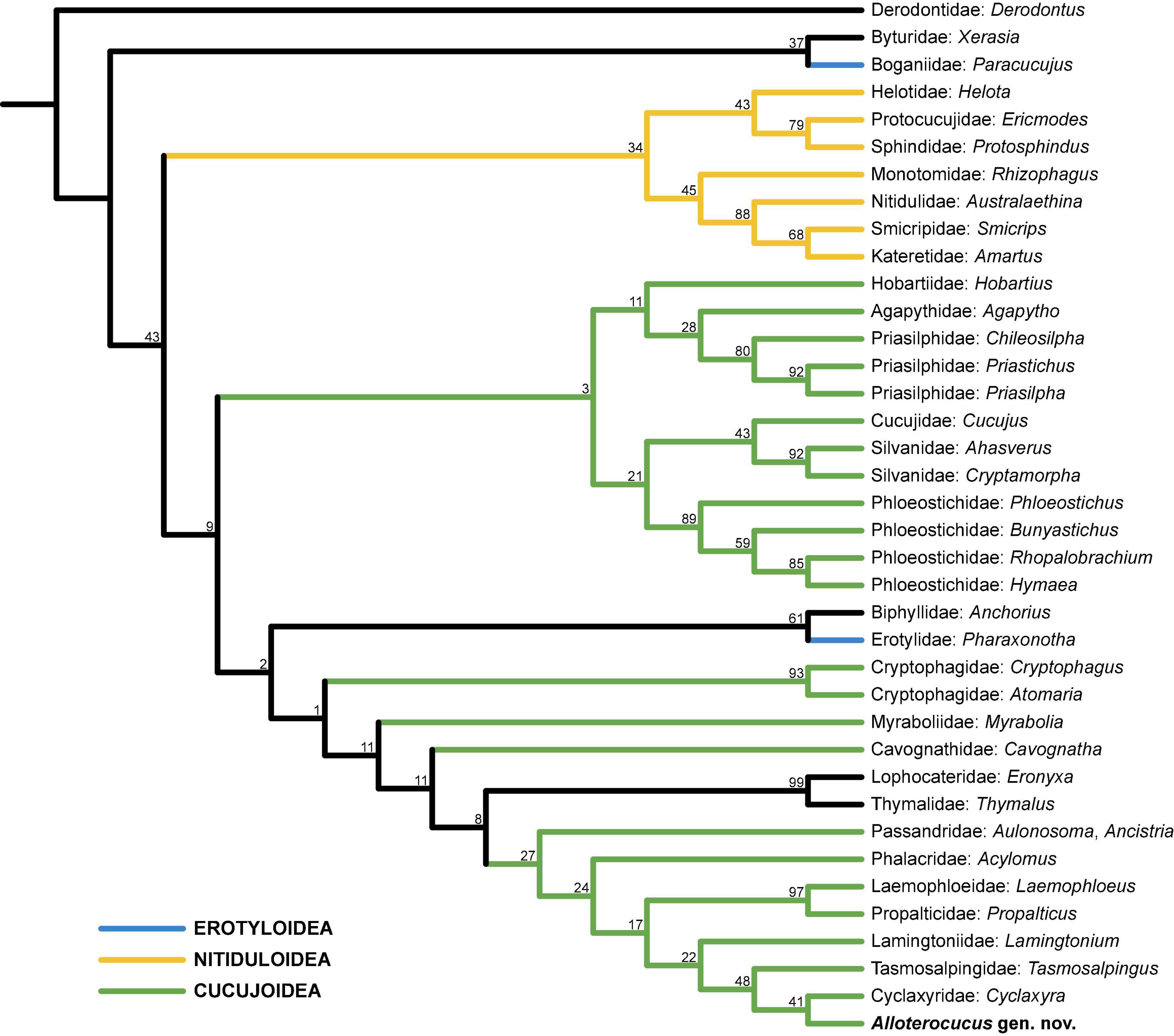
Figure 5. Placement of Alloterocucus Li, Leschen Liu, and Cai gen. nov. based on the single tree resulting from the unconstrained parsimony analysis.
Alloterocucus differs from Tasmosalpingidae, Cyclaxyridae, and Lamingtoniidae by having apically truncate terminal maxillary palpomeres, and the group bears different habitus and individual characters. Cyclaxyridae (Figures 6E,F) is characterized by an ovate and strongly convex body, well-developed antennal grooves, the absence of genal projections, and the presence of epipleural fovea (body elongate, antennal grooves absent, genal projections acute, and epipleural fovea absent in Alloterocucus). Tasmosalpingidae (Figures 6C,D) is somewhat sinuate in outline and is moderately convex. It also has characters including the antennal insertions exposed in dorsal view, clear antennal grooves, confused elytral punctation, and laterally opening setose cavities of the pronotum, which are all absent in Alloterocucus.

Figure 6. Extant representatives of Lamingtoniidae, Tasmosalpingidae, and Cyclaxyridae. (A,B) Lamingtonium loebli Lawrence and Leschen. (C,D) Tasmosalpingus quadrispilotus Lea. (E,F) Cylaxyra politula Broun. Scale bar: 500 μm.
Alloterocucus most closely resembles members of Lamingtoniidae (Figures 6A,B), but lacks distinct dorsal punctation, and the pronotal width is equal to the combined widths of the elytra. If we make some assumptions on the character states of the mandible (i.e., lacking a tubercle, which can be inferred sometimes based on the shape of the clypeus), Alloterocucus can be keyed to couplet 26 in the family-group key provided by Leschen et al. (2005), and since it lacks the distinctive setose cavities on the prothorax of Tasmosalpingus Lea, Alloterocucus can be keyed further to couplet 27 containing Lamingtoniidae and Cucujidae. Apart from the raised supra-ocular carinae and the tarsal formula, which is 5-5-5 in extant Lamingtoniidae, it can be placed into this family and excluded from Cucujidae by having a well-defined antennal club. However, if we were able to see the internal structure of the meso-metaventral articulation, a key feature of this couplet, then we would be able to better place Alloterocucus in Cucujoidea sensu stricto.
Sen Gupta and Crowson (1969) excluded Lamingtoniidae from the cerylonid series (now Coccinelloidea) by the 5-5-5 tarsal formula, and from several families of the Cucujoidea sensu lato based on other characters, arguing that the family is related to Languriidae (now included in Erotylidae), as proposed by Kirejtshuk (2000) but rejected by Lawrence and Leschen (2003) based on larval and adult characters. The open mesocoxal cavities exclude Alloterocucus from Erotylidae, a character shared by most members of the family (but see Yoshida and Leschen, 2020) and support its affinity to Lamingtoniidae. Alloterocucus differs from members of Lamingtonium Sen Gupta and Crowson by several characters, including those mentioned above, but also the absence of curved longitudinal grooves extending backward from the maxillary base (present in Lamingtonium binnaburrense Sen Gupta and Crowson and L. loebli Lawrence and Leschen but absent in L. thayerae Lawrence and Leschen).
Two recent phylogenomic analyses recognized three separate lineages of Cucujoidea sensu lato. Thus, it was divided into three superfamilies by Cai et al. (2022): Erotyloidea, Nitiduloidea, and Cucujoidea sensu stricto. However, the relationships among the traditional cucujoid families are still far from being settled. Many small, rare families were not sampled in the analysis by Cai et al. (2022). McKenna et al. (2019) presented a synthesized tree in their figure 2, with all cucujoid families present, but some of the families (Smicripidae, Cyclaxyridae, Cavognathidae, Agapythidae, and Priasilphidae) were not included in their phylogenetic analysis and were manually inserted into the synthesized tree based on the results of McKenna et al. (2015). McKenna et al. (2015), however, though with a broader taxon sampling, used only eight genes, which may not be sufficient for inferring deeper interfamilial relationships as suggested by the discrepancies between their maximum likelihood and Bayesian inference analyses and low support. The topologies of many groups in McKenna et al. (2015) were largely inconsistent with the recent more comprehensive studies, especially for deeper nodes (McKenna et al., 2019; Cai et al., 2022). Thus, the positions of these families remain questionable until more genetic data are available.
Although Tasmosalpingidae and Lamingtoniidae were placed in the Cucujoidea-3 lineage (Cucujoidea sensu stricto; Cai et al., 2022) by McKenna et al. (2019), no molecular data exist for either family and their current placements are based on morphology. Moreover, the inconsistency between morphological (Leschen et al., 2005; Lawrence et al., 2011) and molecular (McKenna et al., 2019; Cai et al., 2022) phylogenies of Cucujoidea indicates that morphology-based studies may not be sufficient for properly resolving the systematic positions of cucujoid families due mainly to rampant convergences (McElrath et al., 2015). Thus, the superfamilial attribution of Tasmosalpingidae and Lamingtoniidae (as well as Alloterocucus) should still be viewed as contentious. The future inclusion of these families in a phylogenomic study will not only help better understand their phylogenetic relationships but also shed light on the position of the enigmatic fossil genus Alloterocucus.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material. The original confocal data are available in Zenodo repository (doi: 10.5281/zenodo.6717396). Further inquiries can be directed to the corresponding author.
Author contributions
C-YC and Y-DL conceived the study. C-YC and D-YH acquired and processed the specimen. Y-DL and Z-HL acquired and processed the photomicrographs. Y-DL performed the analyses. Y-DL and RABL drafted the manuscript, to which C-YC and Z-HL contributed. All authors commented on the manuscript and gave final approval for publication.
Funding
Financial support was provided by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000), the National Natural Science Foundation of China (42222201 and 42288201), and the Second Tibetan Plateau Scientific Expedition and Research project (2019QZKK0706). Y-DL was supported by a scholarship granted by the China Scholarship Council (202108320010). RABL was funded by Strategic Science Investment Funding for Crown Research Institutes from the Ministry of Business, Innovation, and Employment’s Science and Innovation Group.
Acknowledgments
We are grateful to Rong Huang and Yan Fang for their technical help with confocal imaging. The editor and two reviewers provided helpful comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.972343/full#supplementary-material
References
Bocak, L., Barton, C., Crampton-Platt, A., Chesters, D., Ahrens, D., and Vogler, A. P. (2014). Building the Coleoptera tree-of-life for >8000 species: composition of public DNA data and fit with Linnaean classification. Syst. Entomol. 39, 97–110. doi: 10.1111/syen.12037
Cai, C., Tihelka, E., Giacomelli, M., Lawrence, J. F., Ślipiński, A., Kundrata, R., et al. (2022). Integrated phylogenomics and fossil data illuminate the evolution of beetles. R. Soc. Open Sci. 9:211771. doi: 10.1098/rsos.211771
Crowson, R. A. (1955). The Natural Classification of the Families of Coleoptera. London: Nathaniel Lloyd.
Fikáèek, M., Beutel, R. G., Cai, C., Lawrence, J. F., Newton, A. F., Solodovnikov, A., et al. (2020). Reliable placement of beetle fossils via phylogenetic analyses–Triassic Leehermania as a case study (Staphylinidae or Myxophaga?). Syst. Entomol. 45, 175–187. doi: 10.1111/syen.12386
Fu, Y.-Z., Li, Y.-D., Su, Y.-T., Cai, C.-Y., and Huang, D.-Y. (2021). Application of confocal laser scanning microscopy to the study of amber bioinclusions. Palaeoentomology 4, 266–278. doi: 10.11646/palaeoentomology.4.3.14
Gimmel, M. L., and Leschen, R. A. B. (2022). Revision of the genera of Picrotini (Coleoptera: Cryptophagidae: Cryptophaginae). Acta Entomol. Mus. Natl. Pragae 62, 61–109. doi: 10.37520/aemnp.2022.006
Gimmel, M. L., Szawaryn, K., Cai, C., and Leschen, R. A. B. (2019). Mesozoic sooty mould beetles as living relicts in New Zealand. Proc. R. Soc. B 286:20192176. doi: 10.1098/rspb.2019.2176
Goloboff, P. A., Torres, A., and Arias, J. S. (2018). Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics 34, 407–437. doi: 10.1111/cla.12205
Hunt, T., Bergsten, J., Levkanicova, Z., Papadopoulou, A., John, O. S., Wild, R., et al. (2007). A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science 318, 1913–1916. doi: 10.1126/science.1146954
Kirejtshuk, A. G. (2000). On origin and early evolution of the superfamily Cucujoidea (Coleoptera, Polyphaga). Comments on the family Helotidae. Kharkov Entomol. Soci. Gazette 8, 8–38.
Kirejtshuk, A. G., Willig, C., and Chetverikov, P. E. (2019). Discovery of a new sphindid genus (Coleoptera, Sphindidae, Protosphindinae) in Cretaceous amber of Northern Myanmar and taxonomic notes. Palaeoentomology 2, 602–610. doi: 10.11646/palaeoentomology.2.6.11
Lawrence, J. F., and Leschen, R. A. B. (2003). “Review of Lamingtoniidae (Coleoptera: Cucujoidea) with descriptions of two new species,” in Systematics of Coleoptera: Papers Celebrating the Retirement of Ivan Löbl, eds G. Cuccodoro and R. A. B. Leschen (Florida: International Associated Publishers), 905–919.
Lawrence, J. F., and Newton, A. F. Jr. (1982). Evolution and classification of beetles. Ann. Rev. Ecol. Syst. 13, 261–290. doi: 10.1146/annurev.es.13.110182.001401
Lawrence, J. F., and Ślipiński, A. (2013). Australian Beetles. Volume 1: Morphology, Classification and Keys. Clayton: CSIRO Publishing. doi: 10.1071/9780643097292
Lawrence, J. F., Ślipiński, A., Seago, A. E., Thayer, M. K., Newton, A. F., and Marvaldi, A. E. (2011). Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Ann. Zool. 61, 1–217. doi: 10.3161/000345411X576725
Leschen, R. A. B., Lawrence, J. F., and Ślipiński, S. A. (2005). Classification of basal Cucujoidea (Coleoptera: Polyphaga): cladistic analysis, keys and review of new families. Invert. Syst. 19, 17–73. doi: 10.1071/IS04007
Letunic, I., and Bork, P. (2021). Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296. doi: 10.1093/nar/gkab301
Liu, Z., Ślipiński, A., Wang, B., and Pang, H. (2019). The oldest Silvanid beetles from the Upper Cretaceous Burmese amber (Coleoptera, Silvanidae, Brontinae). Cretac. Res. 98, 1–8. doi: 10.1016/j.cretres.2019.02.002
Mao, Y., Liang, K., Su, Y., Li, J., Rao, X., Zhang, H., et al. (2018). Various amberground marine animals on Burmese amber with discussions on its age. Palaeoentomology 1, 91–103. doi: 10.11646/palaeoentomology.1.1.11
McElrath, T. C., Robertson, J. A., Thomas, M. C., Osborne, J., Miller, K. B., Mchugh, J. V., et al. (2015). A molecular phylogenetic study of Cucujidae s.l. (Coleoptera: Cucujoidea). Syst. Entomol. 40, 705–718. doi: 10.1111/syen.12133
McKenna, D. D., Wild, A. L., Kanda, K., Bellamy, C. L., Beutel, R. G., Caterino, M. S., et al. (2015). The beetle tree of life reveals that Coleoptera survived end-Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst. Entomol. 40, 835–880. doi: 10.1111/syen.12132
McKenna, D. D., Shin, S., Ahrens, D., Balke, M., Beza-Beza, C., Clarke, D. J., et al. (2019). The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. U.S.A. 116, 24729–24737. doi: 10.1073/pnas.1909655116
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Robertson, J. A., Ślipiński, A., Moulton, M., Shockley, F. W., Giorgi, A., Lord, N. P., et al. (2015). Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Syst. Entomol. 40, 745–778. doi: 10.1111/syen.12138
Robertson, J. A., Whiting, M. F., and McHugh, J. V. (2008). Searching for natural lineages within the Cerylonid Series (Coleoptera: Cucujoidea). Mol. Phylogenet. Evol. 46, 193–205. doi: 10.1016/j.ympev.2007.09.017
Sen Gupta, T., and Crowson, R. A. (1969). On a new family of Clavicornia (Coleoptera) and a new genus of Languriidae. Proc. R. Entomol. Soc. Lond. B 38, 125–131. doi: 10.1111/j.1365-3113.1969.tb00245.x
Shi, G., Grimaldi, D. A., Harlow, G. E., Wang, J., Wang, J., Yang, M., et al. (2012). Age constraint on Burmese amber based on U-Pb dating of zircons. Cretac. Res. 37, 155–163. doi: 10.1016/j.cretres.2012.03.014
Slater, G. J. (2013). Phylogenetic evidence for a shift in the mode of mammalian body size evolution at the Cretaceous-Palaeogene boundary. Methods Ecol. Evol. 4, 734–744. doi: 10.1111/2041-210X.12084
Ślipiński, S. A., and Pakaluk, J. (1991). “Problems in the classification of the Cerylonid series of Cucujoidea (Coleoptera),” in Advances in Coleopterology, eds M. Zunino, X. Belles, and M. Blas (Barcelona: European Association of Coleopterology), 79–88.
Smith, M. R. (2019). Bayesian and parsimony approaches reconstruct informative trees from simulated morphological datasets. Biol. Lett. 15:20180632. doi: 10.1098/rsbl.2018.0632
Smith, M. R. (2021). TreeSearch: morphological phylogenetic analysis in R. bioRxiv [Preprint] doi: 10.1101/2021.11.08.467735
Tihelka, E., Huang, D., and Cai, C. (2020). Pleuroceratos jiewenae sp. nov.: A new Cretaceous phloeostichid beetle (Coleoptera: Cucujoidea: Phloeostichidae). Palaeoentomology 3, 248–259. doi: 10.11646/palaeoentomology.3.3.6
Timmermans, M. J. T. N., Barton, C., Haran, J., Ahrens, D., Culverwell, C. L., Ollikainen, A., et al. (2016). Family-level sampling of mitochondrial genomes in Coleoptera: compositional heterogeneity and phylogenetics. Genome Biol. Evol. 8, 161–175. doi: 10.1093/gbe/evv241
Yoshida, T., and Leschen, R. A. B. (2020). A new genus of Pharaxonothinae from Australia (Coleoptera: Erotylidae). Zootaxa 4838, 273–282. doi: 10.11646/zootaxa.4838.2.7
Keywords: Cucujoidea, phylogeny, fossil, Burmese amber, Mesozoic
Citation: Li Y-D, Leschen RAB, Liu Z-H, Huang D-Y and Cai C-Y (2022) An enigmatic beetle with affinity to Lamingtoniidae in mid-Cretaceous amber from northern Myanmar (Coleoptera: Cucujoidea). Front. Ecol. Evol. 10:972343. doi: 10.3389/fevo.2022.972343
Received: 18 June 2022; Accepted: 21 September 2022;
Published: 03 November 2022.
Edited by:
Tae-Yoon Park, Korea Polar Research Institute, South KoreaReviewed by:
Erik Tihelka, University of Bristol, United KingdomVitalii Igorevich Alekseev, Immanuel Kant Baltic Federal University, Russia
Copyright © 2022 Li, Leschen, Liu, Huang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen-Yang Cai, cycai@nigpas.ac.cn
 Yan-Da Li
Yan-Da Li Richard A. B. Leschen4
Richard A. B. Leschen4  Chen-Yang Cai
Chen-Yang Cai