Case report: COVID-19-associated mucormycosis co-infection with Lomentospora prolificans: The first case and review on multiple fungal co-infections during COVID-19 pandemic
- 1Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- 2Department of Infectious Diseases, School of Medicine, Infectious Diseases Research Center, Kashan University of Medical Sciences, Kashan, Iran
- 3Department of Medical Parasitology and Mycology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
- 4Mycology Reference Laboratory, Research Core Facilities Laboratory, Isfahan University of Medical Sciences, Isfahan, Iran
- 5Department of Pathology and Histology, School of Medicine, Shahid Beheshti Hospital, Kashan University of Medical Sciences, Kashan, Iran
- 6Department of Otorhinolaryngology, School of Medicine, Matini Hospital, Kashan University of Medical Sciences, Kashan, Iran
Along with the pandemic COVID-19 spreads, new clinical challenges have emerged in the health care settings, among which there is a high risk of secondary invasive fungal infections with significant mortality. Here, we report a case of invasive fungal rhino orbital sinusitis due to the simultaneous co-infection by Rhizopus oryzae and Lomentospora prolificans, both identified by sequencing, in a 70-year-old Afghanistanian female with COVID-19. The patient was subjected to surgical debridement as well as taking liposomal amphotericin B, voriconazole, and on discharge, her condition was good. As far as we know, this is the first case of co-infection of COVID-19-associated mucormycosis (CAM) and Lomentospora prolificans infection. Multiple fungal co-infections in COVID-19 patients are reviewed.
Introduction
Most COVID-19 patients present with mild or moderate disease, however, patients with severe COVID-19, with comorbidities, or those receiving corticosteroid therapy and/or mechanical ventilation and intensive care, are predisposed to secondary opportunistic fungal infections (1) with high morbidity and mortality rate. Classical risk factors for invasive fungal infections in healthcare settings include prolonged neutropenia, hematologic malignancy, bone marrow, and solid organ transplantation, corticosteroid use, long stay in intensive care units (ICU), and uncontrolled diabetes mellitus (2).
Mucormycosis is an uncommon opportunistic life-threatening aggressive infection in humans and animals that is characterized by extensive angioinvasion that leads to vessel thrombosis and tissue necrosis (3), preventing the penetration of immune cells and antifungal agents to the infection site (4). The infection occurs in immunocompromised patients especially those with uncontrolled diabetes mellitus, neutropenia, immunosuppression, hematological malignancy, and hematopoietic stem cell or solid-organ transplantation (5). Rhizopus is the most common fungal agent of human mucormycosis in most case series, followed by Mucor and Lichtheimia, accounting for 70 to 80% of all mucormycosis cases (6, 7). Infections due to other genera such as Rhizomucor, Apophysomyces, Saksenaea, Cunninghamella, Cokeromyces, and Syncephalastrum are very rare (5).
Lomentospora prolificans (formerly Scedosporium prolificans) is an emerging invasive fungal opportunist that affects immunocompromised patients and even immunocompetent individuals (8), with a predilection for skin, sinuses, lungs, and central nervous system (9). The respiratory tract is considered the main route of entry for lomentosporiosis, but it may also occur via contaminated catheters (10). The infection is difficult to treat and has a high mortality.
There are numerous reports on co-infections of COVID-19 with fungal pathogens, but the complication of two filamentous fungal co-infections are rarely reported. As awareness and early diagnosis of such co-infections are important to initiate appropriate antifungal therapy and to prevent death (11), here, we report a simultaneous multiple co-infection by the filamentous fungi Rhizopus oryzae and Lomentospora prolificans in a COVID-19 patient. To better understand the clinical characteristics of such infrequent symbiosis between human fungal pathogens, we have reviewed the reported lomentosporiosis and the multiple fungal co-infections in COVID-19 patients.
Case report
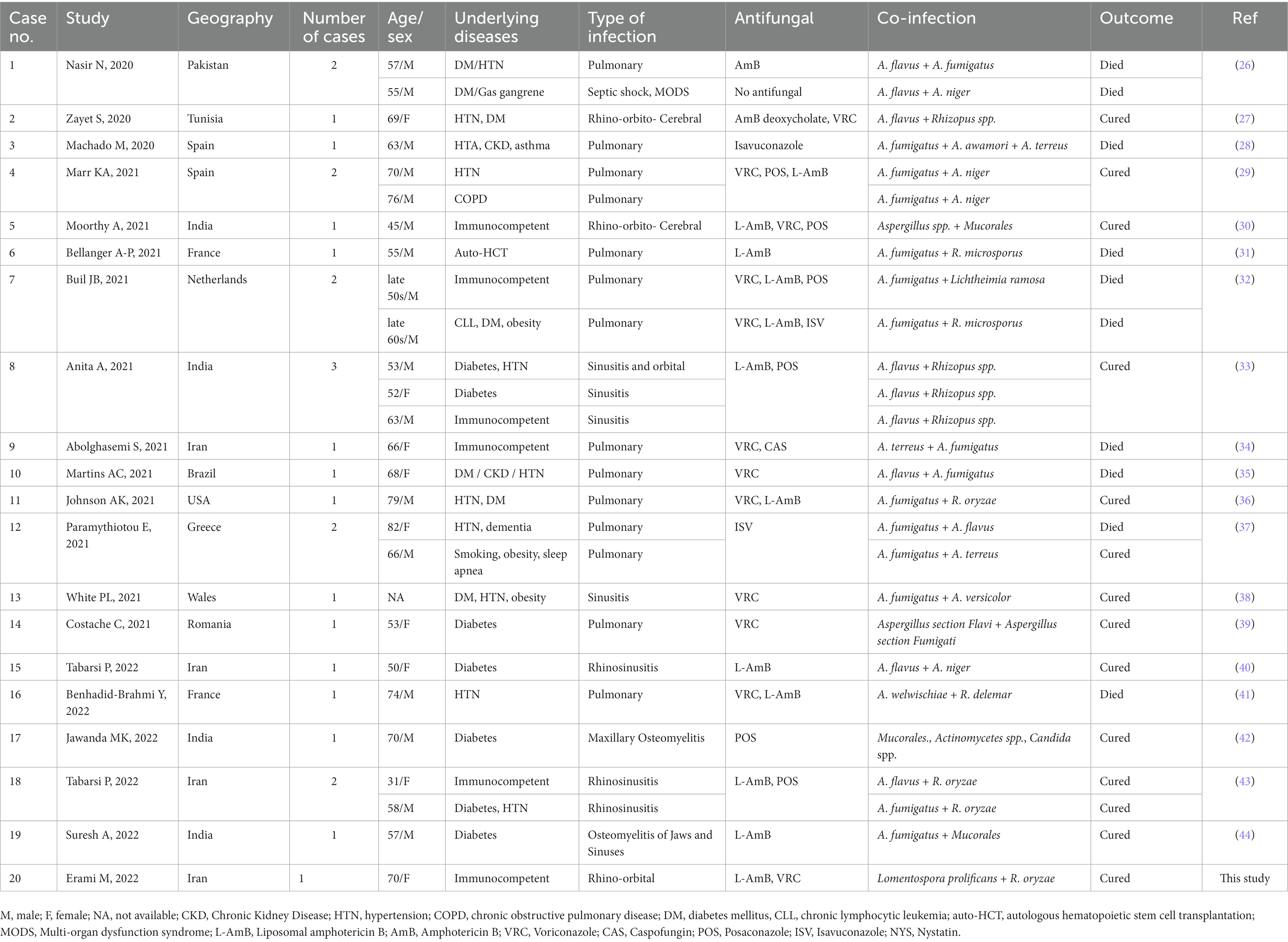
On 29 November 2021 (day 1), a 70-year-old Afghanistanian female suspected of COVID-19 was hospitalized at Shahid-Beheshti Hospital, Kashan, Iran. She had a one-month history of headache, dizziness, dry cough, dyspnea, hemoptysis, myalgia, and severe weakness. In the physical examination, swelling over the right side of her face and numbness of the right side of her upper lip, a body temperature of 36°C, blood pressure of 90/60 mm Hg, respiratory rate of 18 breaths per minute, and oxygen saturation of 90% while the patient was breathing ambient air, were observed. Laboratory investigations were as followed: fasting blood sugar 76 mg/ dL (70–115 mg/dL), hemoglobin A1C 5.6% (Non-diabetic: 4.4–6.7%), sodium 135 mmol/L (135–145 mmol/L), potassium 4.1 mmol/L (3.5–5.3 mmol/L), calcium 8.6 mg/dL (8.6–10.6 mg/dL), blood urea nitrogen 29 mg/dL (7–23 mg/dL), creatinine 1.4 mg/dL (0.4–1.5 mg/dL), erythrocyte sedimentation rate (ESR) 84 mm in the 1st h, hemoglobin 11.6 g/dL (11.7–15.5 g/dL), white cell count 11.7 cells/μL (4–11 cells/μL), lymphocyte count 8.1 cells/μL (18–44 cells/μL), and C-reactive protein (CRP) 130 mg/L (normal <8). Her laboratory blood analysis revealed lymphopenia and a significant increase in CRP. Blood and urine cultures for fungi and bacteria were negative. Nasopharyngeal and oropharyngeal swabs were obtained and subjected to reverse transcription real-time polymerase chain reaction (rt-RT-PCR) for SARS-CoV-2 targeting the N and RdRp genes (Pishtaz Teb, Tehran, Iran) tested by Light Cycler 96 system (Roche, Germany) (12). The sample was positive with a high viral load (Ct value = 14.84 and 13.30 for the two targets). In computed tomography (CT) scan of the chest, bilateral ground-glass opacities with a predominantly peripheral and multilobar location were seen. The largest infiltrate was found in the base of the left lung with a tendency to consolidate, and in the right lung, opacities were located mainly in the upper lobe. Bilateral pleural thickenings were also not reported (Figure 1A).
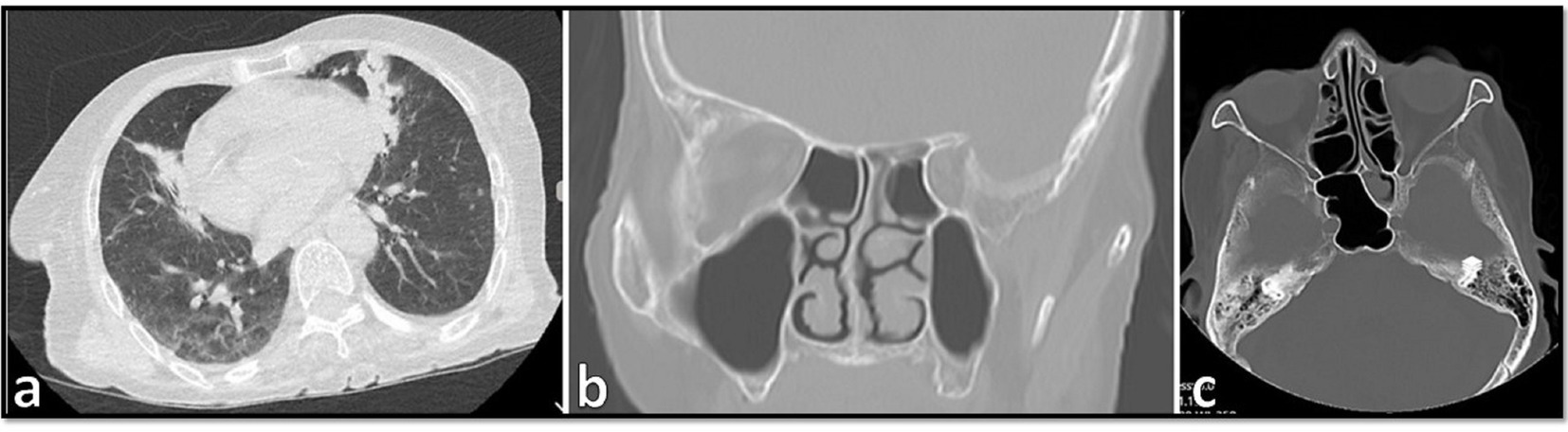
Figure 1. (A) Computed tomography scan of the chest in the case showing bilateral ground-glass opacities with a predominantly peripheral and multilobar location. (B,C) Orbital computed tomography scans of paranasal sinuses demonstrating opacification and air-fluid level in the left sphenoid sinus and some anterior ethmoid sinus air cells in right septal deviation to the right mucosal thickening in the left maxillary sinus.
Since her admission, the patient received atorvastatin (40 mg), levomepromazine (7.5 mL), and valacyclovir (500 mg). After the CT scan, and after the positive PCR results, hydroxychloroquine (400 mg/12 h), remdesivir (100 mg, 6 dosages), azithromycin (500 mg/day), dexamethasone (8 mg intravenously/twice daily) and vancomycin (IV, 1 gr) were added. On day 2, the patient was transferred to the ICU due to the exacerbation of hypoxia and respiratory distress and received mechanical ventilation assistance with orotracheal intubation. Due to the progress of hypoxia and the increase of inflammatory biomarkers, and the onset of the cytokine storm, dexamethasone was stopped and methylprednisolone (125 mg/day) was started for 3 days. After 3 days, the patient developed right periorbital edema and necrosis of the hard palate, and due to the recent increased prevalence of CAM among COVID-19 patients in the hospital, intravenous liposomal amphotericin B (5 mg/kg/day) was started as empirical treatment. CT scans of paranasal sinuses and orbital demonstrated opacification and air-fluid level in the left sphenoid sinus, and some anterior ethmoid sinus air cells in the right septal deviation to the right mucosal thickening in the left maxillary sinus. According to the mucosal thickening and accumulation of secretions in paranasal sinuses (Figures 1B,C), the patient underwent endoscopic sinus and nasal surgery on day 7. Endoscopy revealed black necrotic lesions and tumefaction localized in the right middle meatus. Necrotic lesions were mostly found in the maxillary and the ethmoidal sinuses. Necrotic tissue and a part of its surrounding healthy tissue were removed by surgery.
Although there was not enough evidence that the patient was immunocompromised, enough immunological tests to understand her defense status were not performed, therefore, the immunological status of the patient is unclear.
Samples from sinusitis and tissue necrosis were taken and subjected to direct microscopy using KOH preparation and also to H&E histopathological examination, and broad aseptate and narrower septate hyaline hyphae were seen in both of them (Figures 2A,B), suggestive of proven invasive mucormycosis and hyalohyphomycosis. Therefore, voriconazole 400 mg/12 h on the first day and then 200 mg/12 h were added to the treatment. On day 12, PCR for SARS-CoV-2 was negative, and the same result was obtained again on day 20. Since then, the patient’s respiratory status improved markedly; she had no fever and the leukocyte count was lower than 11 cells/μL. On day 20 and day 23 the patient was discharged from ICU and the hospital, respectively. The patient began a 6-week course of oral voriconazole with close clinical and radiological surveillance. She was followed up for 15 weeks, during which her presenting symptoms have resolved, and is doing well.
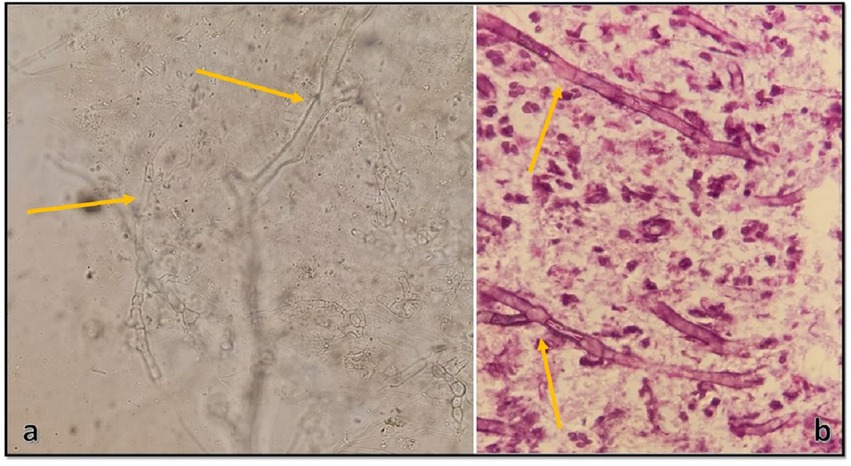
Figure 2. (A) Direct KOH microscopy of tissue samples. Broad aseptate hyphae with wide-angle branches, and narrow septate hyaline hyphae with acute branching, are seen (×400). (B) Histopathological examination of tissue sections revealing a vascular structure involved, and containing both broad and thin fungal hyphae (×1,000).
Tissue samples were cultured on two Sabouraud dextrose agar (Merck, Germany) plates supplemented with 50 mg/L chloramphenicol with three inoculums on each plate, which resulted in the development of two different colonies on both plates. One of the colonies quickly covered the agar surface with dense growth, was cotton candy-like, white at first and then gray or yellowish-brown (Figure 3A), and its reverse was white to pale shades of gray or brown (Figure 3B). In the microscopic study of the slide culture prepared from the pure isolated colonies, broad hyphae with no or very few septa, numerous stolons running among the mycelia, and connecting groups of long sporangiophores were seen (Figures 3C,D). The other isolated colonies were cottony or moist and light gray to black (Figure 4A), and the reverse was gray to black (Figure 4B). The mature colony became dark gray to black and developed white mycelial tufts over time (Figure 4C). In slide culture preparation, septate hyphae, conidiogenous cells (annelids) having a swollen base and elongated neck, and small clusters of olive to brown, one-celled, smooth, and ovoid with a slightly narrowed, truncated base conidia at the apex were observed (Figure 4D). For molecular identification, DNAs of the colonies were extracted using physical destruction by glass-bead manipulation followed by phenol-chloroform purification as described previously (13), and the ITS1-5.8S-ITS2 region was PCR-amplified using the universal primers ITS1 and ITS4 (14). The PCR products were purified and Sanger-sequenced by the forward primer (Core Facilities Laboratory, Isfahan University of Medical Sciences, Iran). Based on NCBI and ISHAM barcoding databases, the isolates were identified as Rhizopus oryzae and Lomentospora prolificans with 100% sequence identity. The sequences were deposited in GenBank under the accession numbers ON220152 and ON220151, respectively.
Figure 3. Surface and reverse of the isolated Rhizopus oryzae colony on Sabouraud dextrose agar after 4 days of incubation at 25°C (A,B), and its microscopic morphology by ×100 and ×400 magnifications, respectively (C,D).
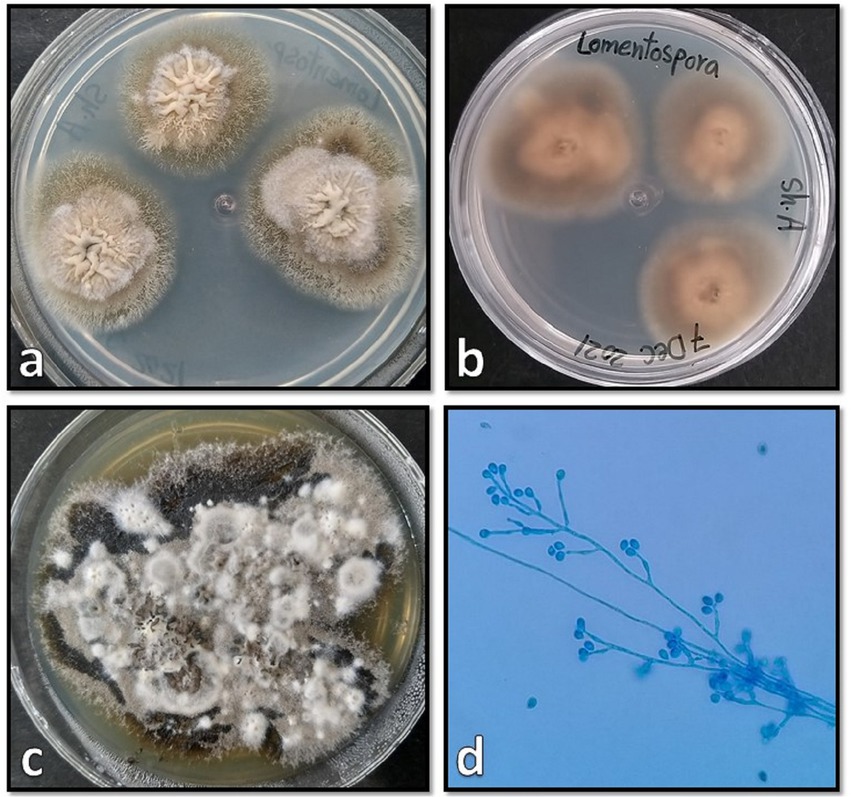
Figure 4. Surface and reverse of the isolated Lomentospora prolificans colony on Sabouraud dextrose agar after 4 days (A,B), and after 20 days (C) incubation at 25°C when the mature colonies became dark gray to black and developed white mycelial tufts, and its microscopic morphology (D).
Antifungal susceptibility testing (AFST) was performed for Rhizopus oryzae and Lomentospora prolificans isolated from the patient, based on the Clinical and Laboratory Standards Institute (CLSI, M38-A2) method for amphotericin B, isavuconazole, posaconazole, itraconazole, voriconazole, fluconazole, caspofungin and terbinafine. The minimum inhibitory concentrations (MIC)/ minimum effective concentration (MEC) were determined visually after 24 and 72 h of incubation, respectively. Candida parapsilosis ATCC 22019 was used as the quality control strain. The results of AFST are seen in Table 1.
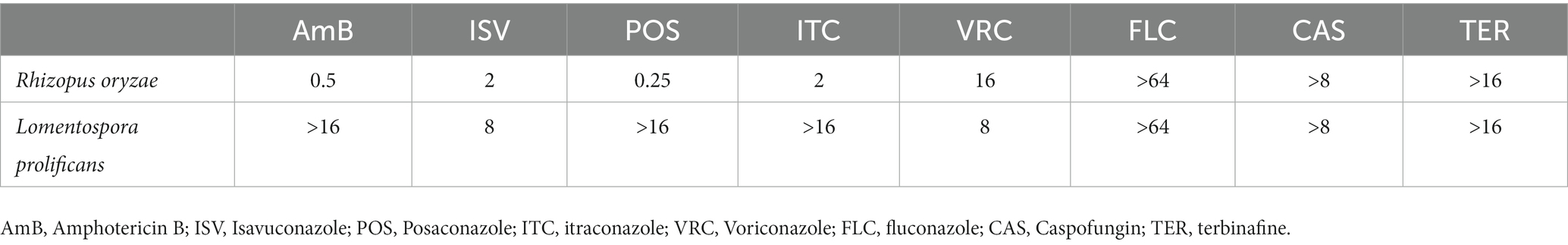
Table 1. The results (μg/mL) of antifungal susceptibility testing of Rhizopus oryzae and Lomentospora prolificans.
Discussion
This is the first case of COVID-associated mucormycosis (CAM) co-infection with Lomentospora prolificans during the global epidemics of SARS-CoV-2. The use of steroids in hospitalized COVID-19 patients and the associated risk of hyperglycemia lead to an increased risk for secondary infections such as invasive aspergillosis and mucormycosis (15). There is an increase in the development of invasive fungal infections in patients who received corticosteroid therapy compared to patients who did not receive steroids (16, 17). In our case, dexamethasone and methylprednisolone were incorporated into cure protocols for COVID-19 infection. The cumulative dose of glucocorticoids was 391 mg. Muthu et al. reported that the cumulative glucocorticoid dose is a contributory factor for CAM. Therefore, in hypoxemic COVID-19 patients, glucocorticoids should be used with caution (16), and should be avoided in patients without hypoxemia (18).
Scedosporium spp./Lomentospora spp., and Fusarium spp. are also emerging rare opportunistic pathogens in immunocompromised individuals with an incidence rate 3–8 times lower than the Mucorales (19). The prognosis of the infections caused by L. prolificans is poor. L. prolificans can be recognized by direct microscopy and culture, however, laboratories are not familiar with its morphology. In addition, in vitro antifungal susceptibility studies confirm that L. prolificans is intrinsically resistant to current available systemic antifungal agents (20) and the disease is often refractory to treatment, yielding high mortality rates of up to 90% (21). It is reported that a combination of terbinafine with miconazole, voriconazole, or itraconazole has synergy against L. prolificans (22). Also, combinations of voriconazole plus either liposomal amphotericin B or micafungin are moderately supported. The ESCMID guidelines (2014) recommended a combination therapy containing voriconazole and terbinafine for L. prolificans infections (23). In a study, five out of eight patients with Lomentospora infections, who received the combination therapy with voriconazole plus at least one other agent (in 4/5 patients the combination included voriconazole and terbinafine), but no patients who received monotherapy, survived (24). In our case, liposomal amphotericin B, voriconazole and surgical debridement were used, and in surgery not only necrotic tissue but also healthy surrounding tissue were removed to prevent spreading to other tissues.
The epidemiology and outcomes of non-Aspergillus invasive mold infections in 3 years between 2014 to 2017 at a university hospital in San Diego, California, United States, were reviewed (24), in which eight cases of Lomentospora prolificans were found, two of them were mixed with Scedosporium apiospermum and Mucor sp. (24). Also, Lamaris et al. (25) reported a case of co-infection with Scedosporium and Rhizopus, but no clinical information is available. The global epidemiological status of lomentosporiosis reported between 2000 to 2018 from more than 74 different countries was reviewed (21), which 56 patients with L. prolificans infection were identified in Australia (n = 14), Japan, the United States (each n = 8), Spain (n = 7), France, Germany (each n = 5), India, Italy, United Kingdom (each n = 2), Brazil, Netherlands, and Poland (each n = 1). Males (n = 32, 57.14%) were more frequently infected than females, ranging from 42 to 67 years. Three out of 56 were mixed with another mold including Aspergillus spp., Exserohilum spp., and Scedosporium apiospermum (21). While L. prolificans is considered an emerging pathogen, it is unknown if it is restricted to some countries in Europe, Australia, and some Southern states of the USA (11) or if the diagnostic conditions have influenced the epidemiology. As far as we know this is the first case of L. prolificans isolated from human specimens in Iran.
There are numerous reports on co-infections of COVID-19 with fungal pathogens, but the complication of multiple filamentous fungal co-infections are rarely reported. As listed in Table 2, fungus-fungus co-infections were mostly observed as pulmonary (n = 14, 51.85%) followed by sinusitis and rhinosinusitis (each n = 3, 10.7%) in which the frequency of Aspergillus-Mucorales (n = 13, 46.42%) and Aspergillus-Aspergillus (n = 12, 42.85%) coexistence was almost similar. The present case is the first report of multiple co-infections of R. oryzae and L. prolificans in COVID-19 patients. We reviewed 27 cases (including the present case) of multiple fungal co-infections published during the COVID-19 pandemic. Only six of the 27 patients were healthy, while the rest were diabetic or had other underlying systemic illnesses. Males (n = 17, 62.96%) were more frequently infected than females and the age range was 31–82 years old, mostly 50–70 years (n = 20, 74.07%). Most patients were treated with amphotericin B, liposomal amphotericin B, or voriconazole; 17 of 27 survived and 10 died among which only two were previously healthy. The clinical overview of patients with multiple fungal co-infections in COVID-19 is summarized in Table 2.
Conclusion
We reported the first case of co-infection of COVID-19-associated mucormycosis with Lomentospora prolificans infection and reviewed the multiple fungal co-infections in COVID-19 patients, and also the cases of lomentosporiosis. Despite frequent prescriptions of broad-spectrum empirical antimicrobials in patients with COVID-19, there is a paucity of data to support the association with two filamentous fungal co-infections. Understanding the predictors of co-infection and multiple infections may help to determine the appropriate interventions to reduce mortality and morbidity. In addition, the generation of prospective evidence to support the detection, identification, development of antimicrobial policy, and appropriate stewardship interventions specific to the COVID-19 pandemic is required.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving human participants were reviewed and approved by ethical approval of the study was obtained from the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.SPH.REC.1399.329). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ME and SA performed all the experiments and participated in data collection. SA drafted the manuscript, participated in database searching and data extraction, and analyzed and interpreted the data. MM-H, SH, AS, AM, and AA participated in collecting the clinical isolate and data collection. HM supervised all parts of the study and critical review of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Isfahan University of Medical Sciences, Isfahan, Iran (grant number 1400180).
Acknowledgments
We gratefully acknowledge to Isfahan University of Medical Sciences, Iran. Also, the authors are grateful to the staff at Shahid-Beheshti Hospital, Kashan, Iran.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lansbury, L, Lim, B, Baskaran, V, and Lim, WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. (2020) 81:266–75. doi: 10.1016/j.jinf.2020.05.046
2. Jeong, W, Keighley, C, Wolfe, R, Lee, WL, Slavin, MA, Chen, SC, et al. Contemporary management and clinical outcomes of mucormycosis: a systematic review and meta-analysis of case reports. Int J Antimicrob Agents. (2019) 53:589–97. doi: 10.1016/j.ijantimicag.2019.01.002
3. Chamilos, G, Lewis, R, Lamaris, G, Walsh, T, and Kontoyiannis, D. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother. (2008) 52:722–4. doi: 10.1128/AAC.01136-07
4. Gebremariam, T, Liu, M, Luo, G, Bruno, V, Phan, QT, Waring, AJ, et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest. (2014) 124:237–50. doi: 10.1172/JCI71349
5. Erami, M, Mirhendi, H, Momen-Heravi, M, Hashemi Hezaveh, SJ, Ahsaniarani, AH, Sabet, SS, et al. A case of COVID-19-associated rhino-orbito-cerebral mucormycosis caused by Apophysomyces variabilis with a review of the literature. Front Cell Infection Microbiol. (2022) 12:898477. doi: 10.3389/fcimb.2022.898477
6. Katragkou, A, Walsh, T, and Roilides, E. Why is mucormycosis more difficult to cure than more common mycoses? Clin Microbiol Infect. (2014) 20:74–81. doi: 10.1111/1469-0691.12466
7. Patel, A, Agarwal, R, Rudramurthy, SM, Shevkani, M, Xess, I, Sharma, R, et al. Multicenter epidemiologic study of coronavirus disease-associated Mucormycosis, India. Emerg Infect Dis. (2021) 27:2349–59. doi: 10.3201/eid2709.210934
8. Cortez, KJ, Roilides, E, Quiroz-Telles, F, Meletiadis, J, Antachopoulos, C, Knudsen, T, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev. (2008) 21:157–97. doi: 10.1128/CMR.00039-07
9. Ramirez-Garcia, A, Pellon, A, Rementeria, A, Buldain, I, Barreto-Bergter, E, Rollin-Pinheiro, R, et al. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol. (2018) 56:S102–25. doi: 10.1093/mmy/myx113
10. Jacobs, SE, Wengenack, NL, and Walsh, TJ. Non-Aspergillus hyaline molds: Emerging causes of sino-pulmonary fungal infections and other invasive mycoses. Semin Respir Crit Care Med. (2020) 41:115–30. doi: 10.1055/s-0039-3401989
11. Al RefaiAl Refaï, M, Duhamel, C, Le Rochais, JP, and Icard, P. Lung scedosporiosis: a differential diagnosis of aspergillosis. Eur J Cardiothorac Surg. (2002) 21:938–9. doi: 10.1016/S1010-7940(02)00068-4
12. Fakhim, H, Nasri, E, Aboutalebian, S, Gholipour, S, Nikaeen, M, Vaezi, A, et al. Asymptomatic carriers of coronavirus disease 2019 among healthcare workers in Isfahan. Iran Fut Virol. (2021) 16:93–8. doi: 10.2217/fvl-2020-0224
13. Aboutalebian, S, Ahmadikia, K, Fakhim, H, Chabavizadeh, J, Okhovat, A, Nikaeen, M, et al. Direct detection and identification of the most common bacteria and fungi causing otitis externa by a stepwise multiplex PCR. Front Cell Infect Microbiol. (2021) 11:210. doi: 10.3389/fcimb.2021.644060
14. Aboutalebian, S, Mahmoudi, S, Mirhendi, H, Okhovat, A, Abtahi, H, and Chabavizadeh, J. Molecular epidemiology of otomycosis in Isfahan revealed a large diversity in causative agents. J Med Microbiol. (2019) 68:918–23. doi: 10.1099/jmm.0.000985
15. Garcia-Vidal, C, Sanjuan, G, Moreno-García, E, Puerta-Alcalde, P, Garcia-Pouton, N, Chumbita, M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. (2021) 27:83–8. doi: 10.1016/j.cmi.2020.07.041
16. Muthu, V, Agarwal, R, Rudramurthy, SM, Thangaraju, D, Shevkani, MR, Patel, AK, et al. Multicenter case-control study of COVID-19-associated Mucormycosis outbreak, India. Emerg Infect Diseas. (2023) 29:8–19. doi: 10.3201/eid2901.220926
17. Tavakolpour, S, Irani, S, Yekaninejad, MS, Alimardi, M, Hasibi, M, Abdollahi, H, et al. Risk factors of COVID-19 associated Mucormycosis (CAM) in Iranian patients: a single-center retrospective study. Mycopathologia. (2022) 187:469–79. doi: 10.1007/s11046-022-00670-5
18. Muthu, V, Sehgal, IS, Dhooria, S, Prasad, KT, Aggarwal, AN, and Agarwal, R. Corticosteroids for non-severe COVID-19: Primum non Nocere. Indian J Crit Care Med. (2022) 26:403–4. doi: 10.5005/jp-journals-10071-24138
19. Park, BJ, Pappas, PG, Wannemuehler, KA, Alexander, BD, Anaissie, EJ, Andes, DR, et al. Invasive non-aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis. (2011) 17:1855–64. doi: 10.3201/eid1710.110087
20. Lackner, M, de Hoog, GS, Verweij, PE, Najafzadeh, MJ, Curfs-Breuker, I, Klaassen, CH, et al. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother. (2012) 56:2635–42. doi: 10.1128/AAC.05910-11
21. Seidel, D, Meißner, A, Lackner, M, Piepenbrock, E, Salmanton-García, J, Stecher, M, et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope®. Crit Rev Microbiol. (2019) 45:1–21. doi: 10.1080/1040841X.2018.1514366
22. Meletiadis, J, Mouton, JW, Meis, JF, and Verweij, PE. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob Agents Chemother. (2003) 47:106–17. doi: 10.1128/AAC.47.1.106-117.2003
23. Tortorano, A, Richardson, M, Roilides, E, Van Diepeningen, A, Caira, M, Munoz, P, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. (2014) 20:27–46. doi: 10.1111/1469-0691.12465
24. Jenks, JD, Reed, SL, Seidel, D, Koehler, P, Cornely, OA, Mehta, SR, et al. Rare mould infections caused by Mucorales, Lomentospora prolificans and Fusarium, in San Diego, CA: the role of antifungal combination therapy. Int J Antimicrob Agents. (2018) 52:706–12. doi: 10.1016/j.ijantimicag.2018.08.005
25. Lamaris, GA, Chamilos, G, Lewis, RE, Safdar, A, Raad, II, and Kontoyiannis, DP. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989–2006. Clin Infect Dis. (2006) 43:1580–4. doi: 10.1086/509579
26. Nasir, N, Farooqi, J, Mahmood, SF, and Jabeen, K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. (2020) 63:766–70. doi: 10.1111/myc.13135
27. Zayet, S, Zaghdoudi, A, Ammari, L, Kilani, B, and Benaissa, HT. Cerebro-rhino-orbital mucormycosis and aspergillosis coinfection in a patient with diabetes mellitus: A case report. IDCases. (2021) 23:e01022. doi: 10.1016/j.idcr.2020.e01022
28. Machado, M, Valerio, M, Álvarez-Uría, A, Olmedo, M, Veintimilla, C, Padilla, B, et al. Invasive pulmonary aspergillosis in the COVID-19 era: an expected new entity. Mycoses. (2021) 64:132–43. doi: 10.1111/myc.13213
29. Marr, KA, Platt, A, Tornheim, JA, Zhang, SX, Datta, K, Cardozo, C, et al. Aspergillosis complicating severe coronavirus disease. Emerg Infect Dis. (2021) 27:18–25. doi: 10.3201/eid2701.202896
30. Moorthy, A, Gaikwad, R, Krishna, S, Hegde, R, Tripathi, K, Kale, PG, et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids—an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofacial Oral Surgery. (2021) 20:418–25. doi: 10.1007/s12663-021-01532-1
31. Bellanger, A-P, Navellou, J-C, Lepiller, Q, Brion, A, Brunel, A-S, Millon, L, et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infectious Diseases Now. (2021) 51:633–5. doi: 10.1016/j.idnow.2021.01.010
32. Buil, JB, van Zanten, AR, Bentvelsen, RG, Rijpstra, TA, Goorhuis, B, van der Voort, S, et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Eur Secur. (2021) 26:2100510. doi: 10.2807/1560-7917.ES.2021.26.23.2100510
33. Anita, A, Kumar, S, Kumari, N, Rajpal, K, Kumar, S, and Singh, RK. A case series of mucormycosis and aspergillus coinfection in post-COVID-19 patient with uncontrolled diabetic. Indian Journal of Case Reports. (2021) 7:420–3. doi: 10.32677/ijcr.v7i10.3054
34. Abolghasemi, S, Hakamifard, A, Sharifynia, S, Pourabdollah Toutkaboni, M, and Azhdari, TH. Fatal invasive pulmonary aspergillosis in an immunocompetent patient with COVID-19 due to Aspergillus terreus: A case study. Clin Case Rep. (2021) 9:2414–8. doi: 10.1002/ccr3.4051
35. Martins, AC, Psaltikidis, EM, de Lima, TC, Fagnani, R, Schreiber, AZ, de Oliveira, CL, et al. COVID-19 and invasive fungal coinfections: A case series at a Brazilian referral hospital. J Med Mycol. (2021) 31:101175. doi: 10.1016/j.mycmed.2021.101175
36. Johnson, AK, Ghazarian, Z, Cendrowski, KD, and Persichino, JG. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. (2021) 32:64–7. doi: 10.1016/j.mmcr.2021.03.006
37. Paramythiotou, E, Dimopoulos, G, Koliakos, N, Siopi, M, Vourli, S, Pournaras, S, et al. Epidemiology and incidence of COVID-19-associated pulmonary aspergillosis (CAPA) in a Greek tertiary care academic reference hospital. Infect Dis Ther. (2021) 10:1779–92. doi: 10.1007/s40121-021-00486-8
38. White, PL, Dhillon, R, Cordey, A, Hughes, H, Faggian, F, Soni, S, et al. A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin Infect Dis. (2021) 73:e1634–44. doi: 10.1093/cid/ciaa1298
39. Costache, C, Botan, A, Mihaila, RM, Colosi, IA, Buksa, SB, and Chiorescu, RM. Mixed etiology COVID-19 associated pulmonary Aspergillosis (CAPA)—a case report and brief review of the literature. J Fungi. (2021) 7:877. doi: 10.3390/jof7100877
40. Tabarsi, P, Sharifynia, S, Toutkaboni, MP, Abtahian, Z, Rahdar, M, Mirahmadian, A, et al. Mixed etiology COVID-19 associated acute rhinosinusitis caused by two Aspergillus species. Ann Med Surgery. (2022) 75:103365. doi: 10.1016/j.amsu.2022.103365
41. Benhadid-Brahmi, Y, Hamane, S, Soyer, B, Mebazaa, A, Alanio, A, Chousterman, B, et al. COVID-19-associated mixed mold infection: a case report of aspergillosis and mucormycosis and a literature review. J Med Mycol. (2022) 32:101231. doi: 10.1016/j.mycmed.2021.101231
42. Jawanda, MK, Narula, R, Gupta, S, Sharma, V, Sidhu, SK, and Kaur, N. Mixed infections (Mucormycosis, Actinomycosis and Candidiasis) leading to maxillary osteomyelitis in a diabetic mellitus patient in post COVID phase: First case report. Acta Med. (2022) 64:218–23. doi: 10.14712/18059694.2022.5
43. Tabarsi, P, Sharifynia, S, Pourabdollah Toutkaboni, M, Abtahian, Z, Rahdar, M, Mirahmadian, AS, et al. Coinfection by Aspergillus and Mucoraceae species in two cases of acute rhinosinusitis as a complication of COVID-19. Case Rep Med. (2022) 2022:1–6. doi: 10.1155/2022/8114388
Keywords: Lomentospora prolificans , COVID-19, mucormycosis, multiple co-infection, antifungal susceptibility testing
Citation: Erami M, Mirhendi H, Momen-Heravi M, Sharif A, Hashemi Hezaveh SJ, Matini AH, Ahsaniarani AH and Aboutalebian S (2023) Case report: COVID-19-associated mucormycosis co-infection with Lomentospora prolificans: The first case and review on multiple fungal co-infections during COVID-19 pandemic. Front. Med. 10:1078970. doi: 10.3389/fmed.2023.1078970
Edited by:
Shisan (Bob) Bao, The University of Sydney, AustraliaReviewed by:
Hector M. Mora-Montes, Universidad de Guanajuato, MexicoSantosh Kumar Swain, Siksha ‘O’ Anusandhan University, India
Valliappan Muthu, Postgraduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2023 Erami, Mirhendi, Momen-Heravi, Sharif, Hashemi Hezaveh, Matini, Ahsaniarani and Aboutalebian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shima Aboutalebian, Shima.Aboutalebian@gmail.com
 Mahzad Erami1,2
Mahzad Erami1,2  Hossein Mirhendi
Hossein Mirhendi Amir Hossein Ahsaniarani
Amir Hossein Ahsaniarani Shima Aboutalebian
Shima Aboutalebian