A Comparative Study of Wheat Leaf Infection by Bipolaris Sorokiniana on Resistant and Susceptible Wheat Varieties using Scanning Electron Microscopy
Md Muzahid E Rahman1, Krishna Kanta Roy2, Kishowar E Mustarin2, Md Sahin Ali2,M.A. Reza2, Md Iqbal Faruk1, Md Sayed Ali1, Pingsheng Ji3 and Md Emran Ali3*
1 Plant Pathology Division, Bangladesh Agricultural Research Institute, Gazipur 1701, Bangladesh
2 Department of Plant Pathology, Bangladesh Wheat and Maize Research Institute, Dinajpur-5200, Bangladesh
3 Department of Plant Pathology, University of Georgia, Tifton, Georgia-31793, USA
- *Corresponding Author:
- Md Emran Ali
Department of Plant Pathology,
University of Georgia,
Tifton,
Georgia,
USA,
E-mail: emran.ali@uga.edu
Received Date:March 10, 2021; Accepted Date: March 24, 2021; ublished Date: March 31, 2021
Citation: Rshman MME, Roy KK, Mustarin KE, Ali MS, et al. (2021) A Comparative Study of Wheat Leaf Infection by Bipolaris sorokiniana on Resistant and Susceptible Wheat Varieties using Scanning Electron Microscopy. Res J Plant Pathol Vol.4 No.S1:001
Abstract
Bipolaris sorokiniana is a phytopathogenic fungus that causes diseases in cerealcrops worldwide. In this study, the speed of infection by the fungus on wheat leafsurface tissues was observed using scanning electron microscopy. The fungus was isolated from diseased wheat plants and identified to be B. sorokiniana based onmorphological characteristics. Young leaves of wheat plants were point inoculatedwith a conidial suspension of B. sorokiniana, and infection process of the pathogenwas observed. The fungus germinated on wheat leaf surface within eight hrs ofinoculation and its germ tube was elongated in 12 hrs of inoculation. On theleaf surface, a hyphal mat was initiated within 24 hrs after inoculation with thinmycelial mat produced by 48 hrs. Moreover, germ tubes elongated more on thesurface of the resistant varieties. The infection rate and pathogenesis depended onhost resistance to the disease. This study provides scanning microscopic evidenceshowing that the fungal pathogen can grow speedily on wheat leaf surface within a short period of time, indicating its virulence on susceptible wheat varieties.
Keywords
Bipolaris sorokiniana ; Infection process; variety selection; Scanning electron microscopy
Introduction
Wheat ( Triicum aesivum )is one of the most consumed cereal food grains. It was the first grain crop grown for 8000 years and is consumed as a staple food in many countries of Europe, West Asia, and Africa [1]. In 2019, world wheat production was around 765 million tons [2]. In Bangladesh, wheat covers 4.53 million hectares of land with a production of 13.75 million tons [3]. The world population is expected to be doubled by the year 2050, so food production needs to increase to feed the ever-growing population [4]. Wheat production in Bangladesh and the world is severely affected by different biotic and abiotic stresses. Diseases play a major role that reduce yield of wheat in Bangladesh as well as in the world. Around 20 different wheat diseases have been reported in Bangladesh [5-8]. Among the various diseases, Bipolaris leaf blight, wheat blast, head blight, black point, loose smut and seedling blight are seed-borne and also the major limiting factors for wheat seed production [9]. Bipolaris leaf blight (or spot blotch), a disease caused by Bipolaris sorokiniana (Sacc.) Shoemaker [teleomorph: Cochliobolus saivus (Ito and Kuribayashi) Drechs. Ex Dastur], affects wheat plants worldwide[10] It is a seed and soil-borne disease and disseminated easily by airborne inocula. This pathogen is one of the most important biotic constrains that limit seed production worldwide. It not only reduces crop yield but is also a source of inoculum resulting in pre and post-emergence death of seedlings, decreased seedling vigor, seed germination and other agronomic traits [11-13]. The disease is becoming more severe due to changes in the cropping systems in many wheat growing areas. It has been reported in most wheat growing areas of India [14], Bangladesh [15], and Nepal [16]. High temperature and high relative humidity favor the outbreak of the disease [17]. The severity of the disease increases with plant age, and disease development becomes faster during grain filling period when high temperature accompanied by high relative humidity prevails [18]. The disease becomes more severe if the crop is lodged during the grain filling period. This fungus mainly attacks at the seedling stage and causes seedling blight, resulting in reduced crop yield. Within 15 days of germination, in inoculated soil the seedling became infected and gradually died with 50%-60% dead plants after 3 weeks due to this disease [19,20] reported that around 50% yield could be reduced due to foot and root rot. The infection of this pathogen on both leaves and roots comprises several phases, i.e. conidial germination, formation of appressorium, penetration, and colonization [21] It produced a phytotoxin prehelminthosporol (C15H24O2) in conidia, hyphae, and the surrounding culture medium that interacts with the host membranes, which caused cell death and leakage of metabolites [22,23] and induced chlorosis and necrosis in plant tissue [24]. Although the infection biology of Bipolaris spp. was well known, little information was available regarding time and speed of infection by the fungus. Therefore, the objective of this study was to determine the speed of conidial germination of Bipolaris sorokiniana during invasion on wheat leaf epidermis.
Materials and Methods
Morphological characteristics of Bipolaris sorokiniana
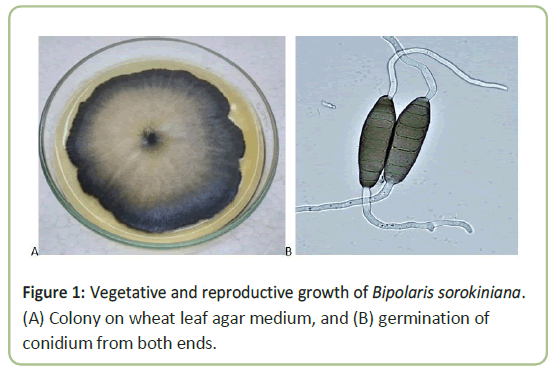
The fungus was identified to be Bipolaris sorokiniana based on vegetative and reproductive growth. The colony color on the upper side was ash to black, and centre of the colony was ash or whitish surrounded by black margin (Figure 1). The underside was dark black. There was a difference in growth and color among some cultures on the petri plates. Colony margin was irregular in shape, with puffy whitish mycelia found over the colony sometimes. Conidiophores were formed singly, straight or flexious, brown to olive brown, while conidia were solitary, straight or slightly flexious, and ellipsoidal tapering, pale brown or slightly black color and germinated from both the ends (Figure 1). The maximum length of conidia was 71.3 mm and the minimum were 38.1 mm, and the difference was statistically significant.
SEM observations
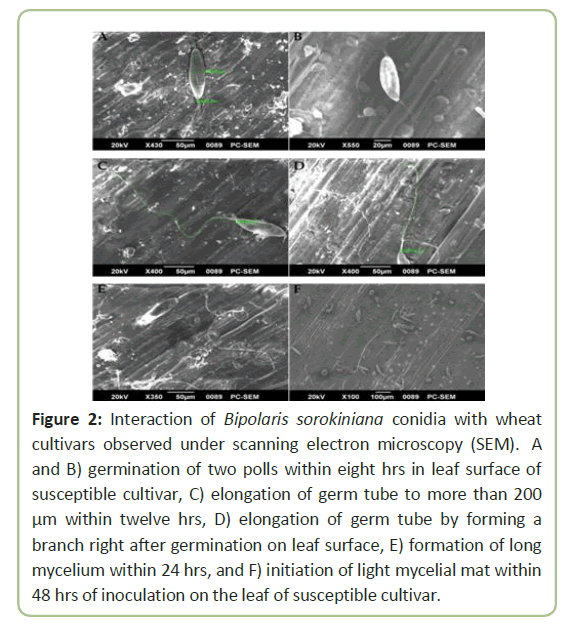
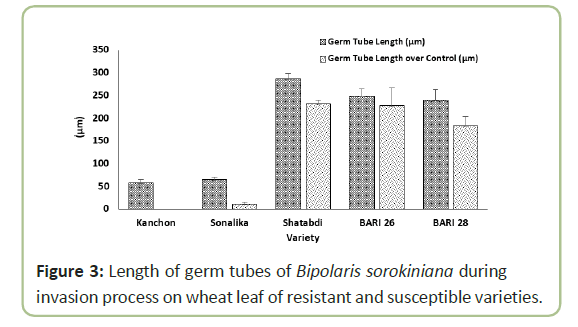
Conidia of Bipolaris sorokiniana germinated and invaded into leaf epidermis successfully under conditions in a moist chamber. After germination, the germ tube elongated and formed or not formed any branch (Figure 2). Germination was observed under both light and electron microscopes, showing germination started within 8 hours of incubation. Germ tube elongation was less in a susceptible variety ‘Kanchon’ than a resistant variety ‘Shatabdi’. In the susceptible variety ‘Kanchon’, germ tube invaded into the epidermis through stomata within eight hours. On the other hand, elongation of germ tube did not enter stomata of the resistant variety ‘Shatabdi’. Highest length of germ tube (286 µm) was observed in ‘Shatabdi’, followed by BARI Gom 26 (248 µm) and BARI Gom 28 (238 µm), and lowest length of germ tube was found in ‘Kanchon’ (56.7 µm) and ‘Sonalika’ (65 µm) (Figure 3).
Figure 2: Interaction of Bipolaris sorokiniana conidia with wheat cultivars observed under scanning electron microscopy (SEM). A and B) germination of two polls within eight hrs in leaf surface of susceptible cultivar, C) elongation of germ tube to more than 200 μm within twelve hrs, D) elongation of germ tube by forming a branch right after germination on leaf surface, E) formation of long mycelium within 24 hrs, and F) initiation of light mycelial mat within 48 hrs of inoculation on the leaf of susceptible cultivar.
Results
Bipolaris sorokiniana can cause different diseases such as common root rot, spot blotch, seedling blight, foot rot, and crown rot of wheat and barley [25]. Spot blotch or Bipolaris leaf blight is one of the most important foliar diseases of wheat in the Indian sub- continent. It is one of the most significant yield-limiting factors for wheat production in many countries like Bangladesh. Mehta reported that B. sorokiniana could cause severe yield losses, up to 100%, in Bangladesh, Bolivia, Brazil, Paraguay and Zambia.
Very limited information is available regarding the infection process of Bipolaris sorokiniana on wheat leaves observed using electron microscopy, although there are reports about infection process of the pathogen. In the case of seedling infection, Sprague (1950) reported that the infection could take place through the stomata on the hypocotyls followed by spreading to the root, shoot and coleoptile. Ultimately, necrotic spots on the leaf indicated air-borne secondary infection and resulted in infected seeds [26]. Domiciano et al. described the infection process of Bipolaris sorokiniana on wheat leaves, which were affected by silicon. Waxy areas of conidial attachment were degraded when the plant was grown with silicon (Si). On the other hand, in the plant without Si, the epidermal wax layer was dissolved followed by the development of fungal hyphae beneath the epidermal layer. It was also reported that dense mycelial mat of hyphae was developed within 96 hours after inoculation, whereas in the present study we observed light mycelial mat within 48 hours in susceptible wheat variety. Similar observation was reported by [27] as engineered rice mutant was unable to uptake Si, which reduced brown spot progress curve in the presence of Si. B. sorokiniana developed interaction with the host cells through destroying host plant tissue by secreting non-specific toxins and production of reactive oxygen species. In addition, the fungus showed remarkable cutinase and esterases activity during pathogenesis. A similar study reported that epicuticular wax of Digitaria sanguinalis significantly increased the growth of germ tubes of Curvularia eragrostidis [28]. According to the report of BWMRI (2018), ‘Kanchon’ and ‘Sonalika’ are susceptible varieties. On the other hand, ‘Shatabdi’ is the most resistant to Bipolaris sorokiniana due to waxiness on the spike, culm and flag leaf sheath [29]. Wheat varieties ‘BARI Gom 28’ and ‘BARI Gom 26’ are moderately resistant to the pathogen. ’ Length of germ tubes of the fungus was shorter on susceptible varieties and longer on resistant varieties during infection, which is in agreement with Agrios (2005). Further studies can be conducted to assess biochemical interaction of the pathogen with the host plants. This study provides scanning microscopic evidence showing that the fungus Bipolaris can grow speedily on wheat leaf surface within a short period of time, indicating its pathogenicity on susceptible wheat varieties. It was also shown that the rate of infection and pathogenesis depended on the degree of host resistance.
Discussion
Collection of diseased samples
Wheat leaves with symptoms of Bipolaris leaf blight (BpLB) were collected from the experimental field of Bangladesh Wheat and Maize Research Institute (BWMRI) in Dinajpur, Bangladesh, in 2017. The cultivar ‘Kanchan’ was highly susceptible to the disease, which was commonly used as a susceptible check in different Bipolaris screening nursery. Leaves were placed in paper bags and brought to the laboratory for isolation of the fungal pathogen.
Incubation of diseased samples
The leaf samples were cut into small pieces (approximately 3-4 cm), surface sterilized with 1% NaOCl for 20-30 seconds, and rinsed with distilled water. The samples were air dried and incubated for 48 hrs on moistened 3-layered Whatman blotter paper in sterilized glass Petri plates. After incubation, lesions on the samples were observed under stereo and compound microscope to confirm the reproductive structure (conidia) of the fungus including septation and length-breadth of conidia.
Inoculum preparation
For culturing the fungus, wheat leaf agar medium was prepared that contained 20 g wheat leaf and 17 g agar in a litre of water. Wheat leaf was boiled in 800 ml of water for 30-40 minutes in a steel saucepan, and the extract was filtered through 2 layers of cheesecloth. Agar powder was added to the extract, boiled for 15 minutes, and distilled water was added to make the volume to 1000 ml before autoclaving. Conidia of the pathogen were taken from sporulating lesions with a sterile needle and transferred onto leaf agar plates. After 7-10 days of incubation at 25°C, colony characteristics of the fungus were evaluated including colony size, color, and margin. In the meantime, mycelia and conidia on the plates were washed with sterile water containing gelatin (1%, w/v) and scraped with a camel hair brush. The suspension was filtered through a 3-layer cheesecloth and placed in a sterile beaker. Tween 20 solution (1-2 drops) was added and the spore suspension was gently mixed for inoculation.
Growth of wheat seedling and inoculation
Wheat seeds were sown in plastic pots (14 x 12 x 8 cm) containing soil and cow dung (1:1 ratio), 10 seeds per pot. The pots were incubated under lab conditions and watered regularly. Wheat leaves were taken 10 days after seedling emergence for inoculation. Ten leaves were cut from the base with sterile scissors and placed on glass slides separately and sealed with a scotch tape at both ends. A volume of 0.20 mL of suspension (104 conidia/mL) was applied to the middle of leaf blades using a micropipette. Immediately after inoculation, leaves were placed in a growth chamber at 25 ± 2 and a relative humidity of 85 ± 2% and checked twice a day for conidial germination observation.
Scanning Electron Microscopy(SEM)
The inoculated areas of the 10 leaf samples were cut into small pieces, upper epidermis was scrapped, and were carefully washed with sodium cacodylate buffer (0.1 M) and post-fixed with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.2) for 2 hrs at room temperature. The specimens were again washed with the same buffer for 10 min (four times) and dehydrated in a series of alcoholic dilutions (30%, 50%, 70% and 100%). The specimens were then air-dried, mounted on metal stubs with the aid of aluminum double-sided tape, and coated with a platinum coating. The samples were observed under JEOL SEM (JSM6490 LA) by operating at 15.2 kV.
Conclusion
In this study, the speed of infection by the fungus on wheat leaf surface tissues was observed using scanning electron microscopy. The fungus was isolated from diseased wheat plants and identified to be B. sorokiniana based on morphological characteristic 0073. Although the infection biology of Bipolaris spp. was well known, little information was available regarding time and speed of infection by the fungus. Therefore, the objective of this study was to determine the speed of conidial germination of Bipolaris sorokiniana during invasion on wheat leaf epidermis.
Author Contributions
Conceptualization, M.M.E.R.; Data curation, M.M.E.R.; Formal analysis, M.M.E.R.; Funding acquisition, M.M.E.R. and M.E.A.; Investigation, M.M.E.R. and M.E.A.; Methodology, M.M.E.R.; Project administration, M.M.E.
Funding
This work was supported by funds from the Plant Molecular Diagnostic Lab, University of Georgia.
Acknowledgments
The authors thank all the members of plant pathology Division , BARI and MDL, UGA for techincal assistance during this study
Conflicts of Interest
The authors declare no conflict of interest.
References
- Curtis BC, Rajaram S, Gómez Macpherson H (2019) Bread wheat: Improvement and production. Food and Agriculture Organization of the United Nations (FAO) FAO Production Yearbook. Food and Agricultural Organization. Rome, Italy
- BBS (2019) Year book of Agricultural Statistic-2018, Bangladesh Bureau of Statistics, Statistics and Informatics Division, Ministry of Planning, Government of the Peopleâ??s Republic of Bangladesh
- Kendall HW, Pimentel D (1994) Constraints on the expansion of the global food supply. Ambio 1: 198-205.
- Talukder MJ. (1974) Plant diseases in Bangladesh. Bangladesh J Agric 1: 61-83.
- Ahmed HU, Hossain MM. (1985) Final report of project crop disease survey and establishment of a herbarium at BARI. Plant Path Divn.BARI, Joydebpur. 16-70
- Ahmed HU ( 1986) Prevailing wheat diseases in Bangladesh. InThird National Wheat Training Workshop, Wheat Research Centre, BARI, Joydebpur, Gazipur. 124-134
- Alam KB, Shaheed MA, Ahmed AU, Malaker PK. (1994) Bipolaris leaf blight (spot blotch) of wheat in Bangladesh. Wheat in Heat-Stressed Environments: Irrigated, Dry Areas and Rice-Wheat Farming Systems. 339-42
- Mobasser S, Jazayeri MR, Khazaei F, Sadeghi L (2012) Wheat seed contamination with seed-borne diseases in cold climatic zone of Iran
- Misra AP. Variability, physiologic specialization and genetics of pathogenicity in graminicolous Helminthosporia affecting cereal crops.
- Cladosporium D (1993) Prevalence of some seedborne fungi on soft white winter wheat seed from Ontario, Canada. Canadian I nventai re Plant des maladies. 73: 143-149
- Sjöberg J ( 2005) Arbuscular mycorrhizal fungi.
- Niaz I, Dawar S.( 2009 ) Detection of seed borne mycoflora in maize (Zea mays L.). Pak. J. Bot. 41: 443-451.
- Sharma AK, Kumar J, Nagarajan S (1996) Report of the Coordinated Experiments : Crop Protection (Pathology and Nematology). All India Coordinated Wheat Improvement Project, Directorate of wheat research, Karnal India. 185.
- Sprague R (1950) Diseases of cereals and grasses in North America. Diseases of cereals and grasses in North America
- Asad S, Iftikhar S, Munir AN, Ahmad I, Ayub N (2007 ) Pathogenic diversity in Bipolaris sorokiniana isolates collected from different wheat growing areas of the Punjab and NWFP of Pakistan. Pak J Bot 39: 2225-2531
- Aggarwal PK, Talukdar KK, Mall RK (2000) Potential yields of rice-wheat system in the Indo-Gangetic plains of India. Facilitation Unit, Rice-Wheat Consortium for the Indo-Gangetic Plains.
- Sprague R (1950) Diseases of cereals and grasses in North America. Diseases of cereals and grasses in North America.
- Chattopadhay SB ( 1953) Root rot and foot rot of wheat caused by Scierotium roifsii Sacc. and Curvularia specilera (bain) Boed-Helm inthosporium letramera Mcknney. Sci. cult. 19: 101-102.
- Herrman T, Wiese MV. (1985) Influence of cultural practices on incidence of foot rot in winter wheat. Plant disease. 69: 948-950.
- Carlson H, Stenram U, Gustafsson M, Jansson HB. (1991) Electron microscopy of barley root infection by the fungal pathogen Bipolaris sorokiniana. Canadian Journal of Botany. 69: 2724-2731.
- Marrè E (1980) Mechanism of action of phytotoxins affecting plasmalemma functions.
- Carlson H, Stenram U, Gustafsson M, Jansson HB (1991) Electron microscopy of barley root infection by the fungal pathogen Bipolaris sorokiniana. Canadian Journal of Botany. 69: 2724-2731.
- Harborne JB (1983) Toxins of plant-fungal interactions. Plant and Fungal Toxins. 1: 762.
- Kumar J, Hückelhoven R, Beckhove U, Nagarajan S, Kogel KH (2001) A compromised Mlo pathway affects the response of barley to the necrotrophic fungus Bipolaris sorokiniana (teleomorph: Cochliobolus sativus) and its toxins. Phytopathology. 91: 127-133
- Vendrig JC (1956) De levenscyclus van Helminthosporium sativum P. K & B op tarwe en gerst. Tijdschrift over Plantenziekten. 62: 30.
- McGhee GC, Guasco J, Bellomo LM, Blumer-Schuette SE, Shane WW, et al. (2011) Genetic analysis of streptomycin-resistant (SmR) strains of Erwinia amylovora suggests that dissemination of two genotypes is responsible for the current distribution of SmR E. amylovora in Michigan. Phytopathology. 101: 182-191.
- Wang F, Zhang P, Qiang S, Zhu YZ, Xu LL (2008) Effects of epicuticular wax from Digitaria sanguinalis and Festuca arundinacea on infection by Curvularia eragrostidis. Australasian Plant Pathology. 37: 43-52.
- Sarker MA, Itohara Y, Hoque M, Uddin SN (2011) Scope and challenges of organic wheat cultivation in Bangladesh. Australian Journal of Crop Science. 5: 1114-1119.
- Rodrigues FA, Vale FX, Korndörfer GH, Prabhu AS, Datnoff LE, et al. (2003) Influence of silicon on sheath blight of rice in Brazil. Crop Protection. 22: 23-29.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences