Bipolaris, Curvularia, Drechslera, Exserohilum, Helminthosporium
Why do laboratory reports group some mold spores and not others on lab analyses reports?
Bipolaris sp., Brachycladium sp., Curvularia sp., Drechslera sp., Exserohilum sp., Helminthosporium sp., have all been used, changed and interchanged with each other. They are used in the Atlas of Clinical Fungi by G.S. Hoog, J. Guarro, J. Gene, M.J. Figueras. Why does this happen? Some names are given by more than one mycologist in two different locations at relatively the same time. Some are just difference of opinions by the mycologists. Other names have evolved with the genetic information at hand.
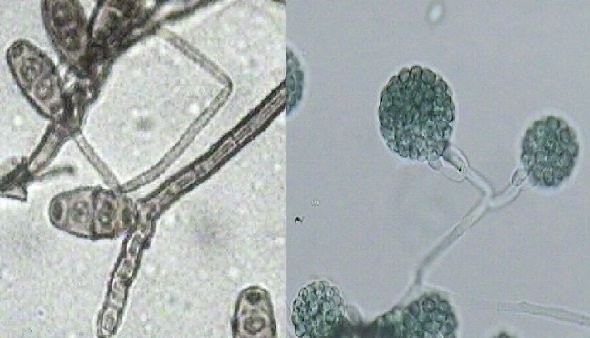
An example of a recent name change is Gliocladium deliquescens to Trichoderma deliquescens. The other name excepted was Gliocladium viride. Everytime I look at this species I see Gliocladium sp. But if I think of Trichoderma species characteristics then I get it. Gliocladium species characteristics are phialides coming off the hyphae forming into a slimy spore ball or mass at the end. Usually one single large slimy spore mass. Trichoderma has phialides coming off the hyphae - arm-like phialides forming slimy spore balls - usually smaller than Gliocladium but still many similar characteristics. So when the genetic testing came back in 2011 and the scientists looked at the relationships there was no doubt but to categorize the once Gliocladium deliquescens or Gliocladium viride to trichoderma deliquescens.
In the laboratory for spore trap analyses the spores often do not give enough information to determine the exact species. The only way to determine the exact spore species is to have genetic testing done. The spore in question then has to have all the genetic work done and close relatives to actually give you the definitive information. Is all this necessary for your client? Most cases the information is not necessary. Only in some cases that deal with health issues such as asthma or allergies or acute conditions will the need be pertinent for definitive information. This can be easily achieved by Anderson impaction testing.
But this does not really answer the question - "Why does the laboratory group some spores and not others?" The similarities of small round spores and the variation from the point the spore is on the conidia to the time it makes it under the microscope causes so much variation that it would be impossible to distinguish what genera the spore came from let alone the species. On occasion the conidia is still attached to the phialide. My reporting technique in this case is to make a note that an Aspergillus species fruiting structure was observed or whatever the case may be.
I think the real answer why laboratories group some spores and not others - say Ascospores versus Cladosporium is a generality. There are hundreds of various ascospores and often they are only picked up outside. Cladosporium is a common fungus. BUT there are many genera that closely resemble Cladosporium - does the lab "need" to differentiate? The answer quite simply is "No, the lab does not need to differentiate." The laboratory has already stipulated that the report is a general report of the indoor or outdoor air quality. Further testing to differentiate the spore types can be done by Anderson impaction. (Or even genetic swab or bulk or other collection procedure predetermined by the testing laboratory) I think most all companies and reports I have worked with give some type of qualifying information to this effect. If they don't it is an implied generalization throughout the laboratory mycological industry.
Independent Business Owner at S & J Property Services, LLC
9yThank you for the post.