Production of Satratoxin G and H Is Tightly Linked to Sporulation in Stachybotrys chartarum
Abstract
:1. Introduction
2. Results
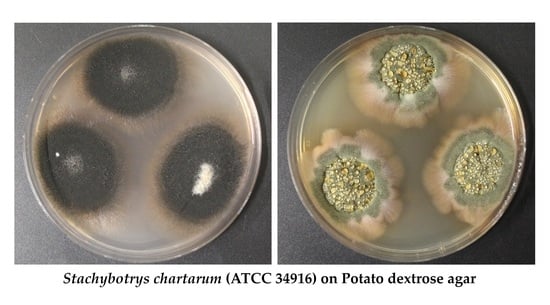
2.1. Colonies Formed under the Different Experimental Conditions
2.2. ATR-IR Measurements
2.3. Mycotoxin Cytotoxicity Assessed with an MTT Assay
2.4. Measurement of Satratoxin G and H Content by HPLC
2.5. Evidence for a Communication between S. chartarum Colonies
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungal Strains and Culture Conditions
5.2. Sample Preparation for Cytotoxicity Assessment and Mycotoxin Analysis
5.3. Sample Preparation for Determination of Spore Count
5.4. Melanin Extraction and ATR-Infrared Spectroscopy
5.5. Cytotoxicity Assessment
5.6. HPLC Measurement and Method Performance
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, B.; Nielsen, K.F.; Jarvis, B.B. Characterization of Stachybotrys from water-damaged buildings based on morphology, growth, and metabolite production. Mycologia 2002, 94, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Biermaier, B.; Gottschalk, C.; Schwaiger, K.; Gareis, M. Occurrence of Stachybotrys chartarum chemotype S in dried culinary herbs. Mycotoxin Res. 2015, 31, 23–32. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.I.; Moubasher, M.H. Toxigenicity and toxins of Stachybotrys isolates from wheat straw samples in Egypt. Exp. Mycol. 1982, 6, 25–30. [Google Scholar] [CrossRef]
- Andersen, B.; Nielsen, K.F.; Thrane, U.; Szaro, T.; Taylor, J.W.; Jarvis, B.B. Molecular and phenotypic descriptions of Stachybotrys chlorohalonata sp. nov. and two chemotypes of Stachybotrys chartarum found in water-damaged buildings. Mycologia 2003, 95, 1227–1258. [Google Scholar] [CrossRef]
- Ulrich, S.; Niessen, L.; Ekruth, J.; Schäfer, C.; Kaltner, F.; Gottschalk, C. Truncated satratoxin gene clusters in selected isolates of the atranone chemotype of Stachybotrys chartarum (Ehrenb.) S. Hughes. Mycotoxin Res. 2020, 36, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, B.B.; Sorenson, W.G.; Hintikka, E.L.; Nikulin, M.; Zhou, Y.; Jiang, J.; Wang, S.; Hinkley, S.; Etzel, R.A.; Dearborn, D. Study of toxin production by isolates of Stachybotrys chartarum and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl. Environ. Microbiol. 1998, 64, 3620–3625. [Google Scholar] [CrossRef] [Green Version]
- Hinkley, S.F.; Moore, J.A.; Squillari, J.; Tak, H.; Oleszewski, R.; Mazzola, E.P.; Jarvis, B.B. New atranones from the fungus Stachybotrys chartarum. Magn. Reson. Chem. 2003, 41, 337–343. [Google Scholar] [CrossRef]
- Hinkley, S.F.; Jiang, J.; Mazzola, E.P.; Jarvis, B.B. Atranones: Novel diterpenoids from the toxigenic mold Stachybotrys atra. Tetrahedron Lett. 1999, 40, 2725–2728. [Google Scholar] [CrossRef]
- Dearborn, D.G.; Smith, P.G.; Dahms, B.B.; Allan, T.M.; Sorenson, W.G.; Montana, E.; Etzel, R.A. Clinical profile of 30 infants with acute pulmonary hemorrhage in Cleveland. Pediatrics 2002, 110, 627–637. [Google Scholar] [CrossRef]
- Miller, J.D.; Rand, T.G.; Jarvis, B.B. Stachybotrys chartarum: Cause of human disease or media darling? Med. Mycol. 2003, 41, 271–291. [Google Scholar] [CrossRef] [Green Version]
- CDC. Pulmonary Hemorrhage/Hemosiderosis among Infants—Cleveland, Ohio, 1993/1996; Morbidity and Mortality Weekly Report; CDC: Atlanta, GA, USA, 2002; pp. 180–184. [Google Scholar]
- Johanning, E.; Biagini, R.; Hull, D.; Morey, P.; Jarvis, B.; Landsbergis, P. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int. Arch. Occup. Environ. Health 1996, 68, 207–218. [Google Scholar] [CrossRef]
- Hintikka, E.-L. The Role of Stachybotrys in the Phenomenon Known as Sick Building Syndrome. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2004; pp. 155–173. [Google Scholar]
- Nikulin, M.; Reijula, K.; Jarvis, B.B.; Hintikka, E.L. Experimental lung mycotoxicosis in mice induced by Stachybotrys atra. Int. J. Exp. Pathol. 1996, 77, 213–218. [Google Scholar] [CrossRef]
- Vesper, S.; Dearborn, D.G.; Yike, I.; Allan, T.; Sobolewski, J.; Hinkley, S.F.; Jarvis, B.B.; Haugland, R.A. Evaluation of Stachybotrys chartarum in the house of an infant with pulmonary hemorrhage: Quantitative assessment before, during, and after remediation. J. Urban Health 2000, 77, 68–85. [Google Scholar] [CrossRef] [Green Version]
- Forgacs, J.; Carll, W.T.; Herring, A.S.; Hinshaw, W.R. Toxicity of Stachybotrys atra for animals. Trans. N. Y. Acad. Sci. 1958, 20, 787–808. [Google Scholar] [CrossRef]
- Schneider, D.J.; Marasas, W.F.; Dale Kuys, J.C.; Kriek, N.P.; Van Schalkwyk, G.C. A field outbreak of suspected Stachybotryotoxicosis in sheep. J. South Afr. Vet. Assoc. 1979, 50, 73–81. [Google Scholar]
- Kriek, N.P.J.; Marasas, W.F.O. Field outbreak of ovine Stachybotryotoxicosis in South Africa. In Thrichothecenes—Chemical, Biological and Toxicological Aspects; Ueno, Y., Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 1983; pp. 279–284. [Google Scholar]
- Semis, M.; Dadwal, S.S.; Tegtmeier, B.R.; Wilczynski, S.P.; Ito, J.I.; Kalkum, M. First Case of Invasive Stachybotrys Sinusitis. Clin. Infect. Dis. 2020, 72, 1386–1391. [Google Scholar] [CrossRef]
- Vesper, S.J.; Magnuson, M.L.; Dearborn, D.G.; Yike, I.; Haugland, R.A. Initial characterization of the hemolysin stachylysin from Stachybotrys chartarum. Infect. Immun. 2001, 69, 912–916. [Google Scholar] [CrossRef] [Green Version]
- Johanning, E.; Landsbergis, P.; Gareis, M.; Yang, C.S.; Olmsted, E. Clinical experience and results of a Sentinel Health Investigation related to indoor fungal exposure. Environ. Health Perspect. 1999, 107, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Hanelt, M.; Gareis, M.; Kollarczik, B. Cytotoxicity of mycotoxins evaluated by the MTT-cell culture assay. Mycopathologia 1994, 128, 167–174. [Google Scholar] [CrossRef]
- Hernández, F.; Cannon, M. Inhibition of protein synthesis in Saccharomyces cerevisiae by the 12,13-epoxytrichothecenes trichodermol, diacetoxyscirpenol and verrucarin A. Reversibility of the effects. J. Antibiot. 1982, 35, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 2005, 22, 369–378. [Google Scholar] [CrossRef]
- Ueno, Y. Trichothecenes—Chemical, Biological and Toxicological Aspects; Elsevier Science Publisher: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Karunasena, E.; Larranaga, M.D.; Simoni, J.S.; Douglas, D.R.; Straus, D.C. Building-associated neurological damage modeled in human cells: A mechanism of neurotoxic effects by exposure to mycotoxins in the indoor environment. Mycopathologia 2010, 170, 377–390. [Google Scholar] [CrossRef]
- Ulrich, S.; Schäfer, C. Toxin Production by Stachybotrys chartarum Genotype S on Different Culture Media. J. Fungi 2020, 6, 159. [Google Scholar] [CrossRef]
- Tag, A.; Hicks, J.; Garifullina, G.; Ake, C., Jr.; Phillips, T.D.; Beremand, M.; Keller, N. G-protein signalling mediates differential production of toxic secondary metabolites. Mol. Microbiol. 2000, 38, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.A.; Schrevens, S.; van Dijck, P.; Goldman, G.H. Fungal G-protein-coupled receptors: Mediators of pathogenesis and targets for disease control. Nat. Microbiol. 2018, 3, 402–414. [Google Scholar] [CrossRef]
- Brodhagen, M.; Keller, N.P. Signalling pathways connecting mycotoxin production and sporulation. Mol. Plant Pathol. 2006, 7, 285–301. [Google Scholar] [CrossRef]
- Gessler, N.N.; Filippovich, S.Y.; Bachurina, G.P.; Kharchenko, E.A.; Groza, N.V.; Belozerskaya, T.A. Oxylipins and oxylipin synthesis pathways in fungi. Appl. Biochem. Microbiol. 2017, 53, 628–639. [Google Scholar] [CrossRef]
- Guo, Y.; Jud, W.; Weikl, F.; Ghirardo, A.; Junker, R.R.; Polle, A.; Benz, J.P.; Pritsch, K.; Schnitzler, J.-P.; Rosenkranz, M. Volatile organic compound patterns predict fungal trophic mode and lifestyle. Commun. Biol. 2021, 4, 673. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed; Crous, P.W., Samson, R.A., Eds.; Westerdijk Laboratory Manual Series; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2019; Volume 2, p. 481. [Google Scholar]
- Ulrich, S.; Biermaier, B.; Bader, O.; Wolf, G.; Straubinger, R.K.; Didier, A.; Sperner, B.; Schwaiger, K.; Gareis, M.; Gottschalk, C. Identification of Stachybotrys spp. by MALDI-TOF mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 7565–7581. [Google Scholar] [CrossRef]
- Marsh, P.B.; Bollenbacher, K. The Vitamin requirements of memnoniella and Stachybotrys. Am. J. Bot. 1946, 33, 245–249. [Google Scholar] [CrossRef]
- Pinto, L.; Granja, L.F.Z.; Almeida, M.A.; Alviano, D.S.; Silva, M.H.D.; Ejzemberg, R.; Rozental, S.; Alviano, C.S. Melanin particles isolated from the fungus Fonsecaea pedrosoi activates the human complement system. Memórias Do Inst. Oswaldo Cruz 2018, 113, e180120. [Google Scholar] [CrossRef] [PubMed]
- Ribera, J.; Panzarasa, G.; Stobbe, A.; Osypova, A.; Rupper, P.; Klose, D.; Schwarze, F. Scalable Biosynthesis of Melanin by the Basidiomycete Armillaria cepistipes. J. Agric. Food Chem. 2019, 67, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Hinkley, S.F.; Jarvis, B.B. Chromatographic Method for Stachybotrys Toxins. In Mycotoxin Protocols; Trucksess, M.W., Pohland, A.E., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 173–194. [Google Scholar]
- Aleksic, B.; Bailly, S.; Draghi, M.; Pestka, J.J.; Oswald, I.P.; Robine, E.; Bailly, J.D.; Lacroix, M.Z. Production of four macrocyclic trichothecenes by Stachybotrys chartarum during its development on different building materials as measured by UPLC-MS/MS. Build. Environ. 2016, 106, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.H. Functional role of secondary metabolites. In Discovering Drugs in Nature (8-9.12.94); IBC Technical Service: London, UK, 1994. [Google Scholar]
- Nielsen, K.F.; Holm, G.; Uttrup, L.P.; Nielsen, P.A. Mould growth on building materials under low water activities. Influence of humidity and temperature on fungal growth and secondary metabolism. Int. Biodeterior. Biodegrad. 2004, 54, 325–336. [Google Scholar] [CrossRef]
- Lozowicka, B.; Iwaniuk, P.; Konecki, R.; Kaczynski, P.; Kuldybayev, N.; Dutbayev, Y. Impact of Diversified Chemical and Biostimulator Protection on Yield, Health Status, Mycotoxin Level, and Economic Profitability in Spring Wheat (Triticum aestivum L.) Cultivation. Agronomy 2022, 12, 258. [Google Scholar] [CrossRef]
- Bata, A.; Vanyi, A.; Lepom, P.; Dashek, W.V.; Llewellyn, G.C. Temperature-Dependent Toxin Production by Stachybotrys Species. In Biodeterioration Research 2; O’Rear, C., Llewellyn, G., Eds.; Springer: New York, NY, USA, 1989; pp. 393–400. [Google Scholar]
- Beever, R.E.; Bollard, E.G. The Nature of the Stimulation of Fungal Growth by Potato Extract. Microbiology 1970, 60, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Jagels, A.; Stephan, F.; Ernst, S.; Lindemann, V.; Cramer, B.; Hubner, F.; Humpf, H.U. Artificial vs. Natural Stachybotrys Infestation—Comparison of Mycotoxin Production on Various Building Materials. Indoor Air 2020, 30, 1268–1282. [Google Scholar] [CrossRef]
- Brzonkalik, K.; Herrling, T.; Syldatk, C.; Neumann, A. The influence of different nitrogen and carbon sources on mycotoxin production in Alternaria alternata. Int. J. Food Microbiol. 2011, 147, 120–126. [Google Scholar] [CrossRef]
- Medina, Á.; Mateo, E.M.; Valle-Algarra, F.M.; Mateo, F.; Mateo, R.; Jiménez, M. Influence of nitrogen and carbon sources on the production of ochratoxin A by ochratoxigenic strains of Aspergillus spp. isolated from grapes. Int. J. Food Microbiol. 2008, 122, 93–99. [Google Scholar] [CrossRef]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef] [Green Version]
- Shim, W.-B.; Woloshuk, C.P. Nitrogen repression of fumonisin B1 biosynthesis in Gibberella fujikuroi. FEMS Microbiol. Lett. 1999, 177, 109–116. [Google Scholar] [CrossRef]
- Hicks, J.K.; Yu, J.-H.; Keller, N.P.; Adams, T.H. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA Gα protein-dependent signaling pathway. EMBO J. 1997, 16, 4916–4923. [Google Scholar] [CrossRef]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [Green Version]
- McDonald, T.; Devi, T.; Shimizu, K.; Sim, S.; Keller, N. Signaling events connecting mycotoxin biosynthesis and sporulation in Aspergillus and Fusarium spp. JSM Mycotoxins 2004, 2003 (Suppl. 3), 139–147. [Google Scholar] [CrossRef] [Green Version]
- Semeiks, J.; Borek, D.; Otwinowski, Z.; Grishin, N.V. Comparative genome sequencing reveals chemotype-specific gene clusters in the toxigenic black mold Stachybotrys. BMC Genom. 2014, 15, 590. [Google Scholar] [CrossRef] [Green Version]
- Buskirk, A.D.; Hettick, J.M.; Chipinda, I.; Law, B.F.; Siegel, P.D.; Slaven, J.E.; Green, B.J.; Beezhold, D.H. Fungal pigments inhibit the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis of darkly pigmented fungi. Anal. Biochem. 2011, 411, 122–128. [Google Scholar] [CrossRef]
- Butt, Z.L.; Ghaffar, A. Inhibition of fungi, actinomycetes and bacteria by Stachybotrys atra. Mycopathol. Mycol. Appl. 1972, 47, 241–251. [Google Scholar] [CrossRef]
- Gregory, L.; Pestka, J.J.; Dearborn, D.G.; Rand, T.G. Localization of satratoxin-G in Stachybotrys chartarum spores and spore-impacted mouse lung using immunocytochemistry. Toxicol. Pathol. 2004, 32, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Nirenberg, H. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitt. Biol. Bundeanstalt Land- Forstwirtsch. 1976, 169, 117. [Google Scholar]
- Gadd, G. Effects of media composition and light on colony differentiation and melanin synthesis in Microdochium bolleyi. Trans. Br. Mycol. Soc. 1982, 78, 115–122. [Google Scholar] [CrossRef]






| Colony Area | SG | SH | SG + SH | SG | SH | SG + SH | |
|---|---|---|---|---|---|---|---|
| cm2 | µg/Agar Plate | µg/cm2 | |||||
| ATCC 34916 PDA-V 3-point culture | 51.5 ± 3.6 | 293.0 ± 6.2 | 488.4 ± 8.8 | 781.3 ± 14.9 | 5.7 ± 0.3 | 9.5 ± 0.5 | 15.2 ± 0.8 |
| IBT 40293 PDA-V 3-point culture | 48.7 ± 2.4 | 346.8 ± 11.6 | 579.0 ± 36.0 | 925.8 ± 47.6 | 7.1 ± 0.1 | 11.9 ± 0.2 | 19.0 ± 0.1 |
| DSM 114129 PDA-V 3-point culture | 46.4 ± 1.7 | 225.0 ± 6.9 | 438.4 ± 4.5 | 663.4 ± 5.9 | 4.9 ± 0.1 | 9.5 ± 0.5 | 14.3 ± 0.5 |
| ATCC 34916 PDA-S 3-point culture | 35.9 ± 1.1 | 38.5 ± 6.7 | 65.2 ± 2.2 | 103.7 ± 4.9 | 1.1 ± 0.2 | 1.8 ± 0.1 | 2.9 ± 0.1 |
| IBT 40293 PDA-S 3-point culture | 38.3 ± 3.8 | 53.2 ± 1.1 | 111.6 ± 33.8 | 164.8 ± 34.8 | 1.4 ± 0.1 | 2.9 ± 0.7 | 4.2 ± 0.5 |
| DSM 114129 PDA-S 3-point culture | 33.7 ± 2.3 | 37.8 ± 5.9 | 80.4 ± 11.5 | 118.3 ± 15.8 | 1.1 ± 0.1 | 2.4 ± 0.3 | 3.5 ± 0.4 |
| ATCC 34916 PDA-V 1-point culture | 17.3 ± 0.4 | 137.2 ± 2.2 | 223.2 ± 1.3 | 360.4 ± 1.9 | 7.9 ± 0.1 | 12.9 ± 0.3 | 20.8 ± 0.4 |
| IBT 40293 PDA-V 1-point culture | 20.8 ± 0.8 | 132.7 ± 20.1 | 215.9 ± 16.5 | 348.6 ± 36.3 | 6.4 ± 0.8 | 10.4 ± 0.4 | 16.7 ± 1.2 |
| DSM 114129 PDA-V 1-point culture | 16.9 ± 0.4 | 71.2 ± 8.4 | 118.1 ± 4.5 | 189.3 ± 10.2 | 4.2 ± 0.4 | 7.0 ± 0.3 | 11.2 ± 0.3 |
| ATCC 34916 PDA-S 1-point culture | 9.3 ± 0.0 | 2.6 ± 1.1 * | n.d. | 2.6 ± 1.1 | 0.3 ± 0.1 | n.d. | 0.3 ± 0.1 |
| IBT 40293 PDA-S 1-point culture | 6.7 ± 0.3 | 12.2 ± 1.1 | 14.5 ± 4.5 | 26.7 ± 4.4 | 1.8 ± 0.2 | 2.2 ± 0.6 | 4.0 ± 0.4 |
| DSM 114129 PDA-S 1-point culture | 8.8 ± 0.3 | 12.2 ± 1.1 * | n.d. | 12.2 ± 1.1 | 1.4 ± 0.1 | n.d. | 1.4 ± 0.1 |
| Mycelium (Wet Weight) | SG | SH | Spore Count | |
|---|---|---|---|---|
| mg | µg/Flask | Spores/Flask | ||
| ATCC 34916 | 1203.3 ± 50.3 | n.d. | n.d. | n.d. |
| IBT 40293 | 1070.0 ± 60.8 | n.d. | n.d. | n.d. |
| DSM 114129 | 1500.0 ± 52.9 | n.d. | n.d. | n.d. |
| Isolate | Culture Medium | Culture Type | Dilution Steps * |
|---|---|---|---|
| IBT 40293 | PDA-V | three-point culture | 17 |
| one-point culture | 15 | ||
| PDA-S | three-point culture | 13 | |
| one-point culture | 11 | ||
| Potato dextrose broth ** | fluid | 5 | |
| ATCC 34916 | PDA-V | three-point culture | 17 |
| one-point culture | 14 | ||
| PDA-S | three-point culture | 14 | |
| one-point culture | 7 | ||
| Potato dextrose broth ** | fluid | 6 | |
| DSM 114129 | PDA-V | three-point culture | 17 |
| one-point culture | 15 | ||
| PDA-S | three-point culture | 12 | |
| one-point culture | 8 | ||
| Potato dextrose broth ** | fluid | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tribelhorn, K.; Twarużek, M.; Soszczyńska, E.; Rau, J.; Baschien, C.; Straubinger, R.K.; Ebel, F.; Ulrich, S. Production of Satratoxin G and H Is Tightly Linked to Sporulation in Stachybotrys chartarum. Toxins 2022, 14, 515. https://doi.org/10.3390/toxins14080515
Tribelhorn K, Twarużek M, Soszczyńska E, Rau J, Baschien C, Straubinger RK, Ebel F, Ulrich S. Production of Satratoxin G and H Is Tightly Linked to Sporulation in Stachybotrys chartarum. Toxins. 2022; 14(8):515. https://doi.org/10.3390/toxins14080515
Chicago/Turabian StyleTribelhorn, Katharina, Magdalena Twarużek, Ewelina Soszczyńska, Jörg Rau, Christiane Baschien, Reinhard K. Straubinger, Frank Ebel, and Sebastian Ulrich. 2022. "Production of Satratoxin G and H Is Tightly Linked to Sporulation in Stachybotrys chartarum" Toxins 14, no. 8: 515. https://doi.org/10.3390/toxins14080515