New Species of Bioluminescent Mycena Sect. Calodontes (Agaricales, Mycenaceae) from Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Morphological Study
2.3. Extraction, Amplification, and Sequencing
2.4. Edition, Alignment, and Phylogenetic Analysis
3. Results
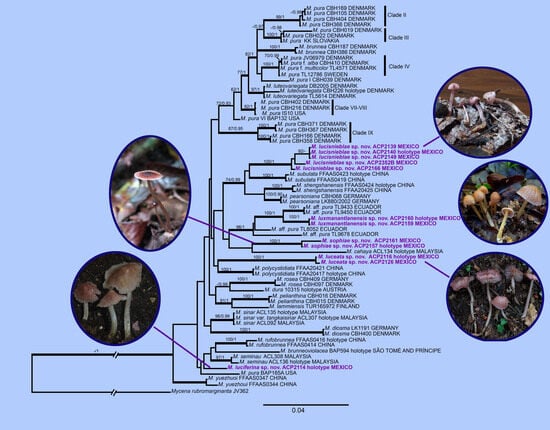
3.1. Phylogenetic Analysis
3.2. Taxonomy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chew, A.L.C.; Tan, Y.S.; Desjardin, D.E.; Musa, M.Y.; Sabaratnam, V. Four new bioluminescent taxa of Mycena sect. Calodontes from Peninsular Malaysia. Mycologia 2014, 106, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Dauner, L.A.P.; Karunarathna, S.C.; Tibpromma, S.; Xu, J.; Mortimer, P.E. Bioluminescent fungus Roridomyces viridiluminus sp. nov. and the first Chinese record of the genus Roridomyces, from Southwestern China. Phytotaxa 2021, 487, 233–250. [Google Scholar] [CrossRef]
- Desjardin, D.E.; Oliveira, A.G.; Stevani, C.V. Fungi bioluminescence revisited. Photochem. Photobiol. Sci. 2008, 7, 170–182. [Google Scholar] [CrossRef]
- Desjardin, D.E.; Perry, B.A.; Stevani, C.V. New luminescent mycenoid fungi (Basidiomycota, Agaricales) from São Paulo State, Brazil. Mycologia 2016, 108, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, S.C.; Mortimer, P.E.; Tibpromma, S.; Dutta, A.K.; Paloi, S.; Hu, Y.; Baurah, G.; Axford, S.; Marciniak, C.; Luangharn, T.; et al. Roridomyces phyllostachydis (Agaricales, Mycenaceae), a new bioluminescent fungus from Northeast India. Phytotaxa 2020, 459, 155–167. [Google Scholar] [CrossRef]
- Oba, Y.; Hosaka, K. The luminous fungi of Japan. J. Fungi 2023, 9, 615. [Google Scholar] [CrossRef]
- Weinstein, P.; Delean, S.; Wood, T.; Austin, A.D. Bioluminescence in the ghost fungus Omphalotus nidiformis does not attract potential spore dispersing insects. IMA Fungus 2016, 7, 229–234. [Google Scholar] [CrossRef]
- Matheny, P.B.; Curtis, J.M.; Hofstetter, V.; Aime, M.C.; Moncalvo, J.M.; Ge, Z.W.; Yang, Z.L.; Slot, J.C.; Ammirati, J.F.; Baroni, T.J.; et al. Major clades of Agaricales: A multilocus phylogenetic overview. Mycologia 2006, 98, 982–995. [Google Scholar] [CrossRef]
- Nimalrathna, T.S.; Tibpromma, S.; Nakamura, A.; Galappaththi, M.C.A.; Xu, J.; Mortimer, P.E.; Karunarathna, S.C. The case of the missing mushroom: A novel bioluminescent species discovered within Favolaschia in southwestern China. Phytotaxa 2022, 539, 244–256. [Google Scholar] [CrossRef]
- Oliveira, J.J.S.; Vargas-Isla, R.; Cabral, T.S.; Cardoso, J.S.; Andriolli, F.S.; Rodrigues, D.P.; Ikeda, T.; Clement, C.R.; Ishikawa, N.K. The Amazonian luminescent Mycena cristinae sp. nov. from Brazil. Mycoscience 2021, 62, 395–405. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.Y.; Lin, W.W.; Kao, H.W. Mycena jingyinga, Mycena luguensis, and Mycena venus: Three new species of bioluminescent fungi from Taiwan. Taiwania 2020, 65, 396–406. [Google Scholar] [CrossRef]
- Aravindakshan, D.M.; Manimohan, P. Mycenas of Kerala; SporePrint Books: Callicut, India, 2015; 213p. [Google Scholar]
- Aronsen, A.; Læssøe, T. The genus Mycena s.l. In The Fungi of Northern Europe; Svampetryk: Copenhagen, Denmark, 2016; Volume 5, 373p. [Google Scholar]
- Cooper, A.C.; Desjardin, D.E.; Perry, B.A. The genus Mycena (Basidiomycota, Agaricales, Mycenaceae) and allied genera from Republic of São Tomé and Príncipe, West Africa. Phytotaxa 2018, 383, 1–47. [Google Scholar] [CrossRef]
- Grgurinovic, C.A. The genus Mycena in South-Eastern Australia. Fungal Divers. Res. Ser. 2003, 9, 1–329. [Google Scholar]
- Harder, C.B.; Læssøe, T.; Kjøller, R.; Frøslev, T.G. A comparison between ITS phylogenetic relationships and morphological species recognition within Mycena sect. Calodontes in Northern Europe. Mycol. Prog. 2010, 9, 395–405. [Google Scholar] [CrossRef]
- Harder, C.B.; Lodge, D.J.; Petersen, R.H.; Hughes, K.W.; Cifuentes Blanco, J.; Frøslev, T.G.; Læssøe, T. Amyloidity is not diagnostic for species in the Mycena pearsoniana complex (Mycena sectio Calodontes). Mycol. Prog. 2012, 11, 725–732. [Google Scholar] [CrossRef]
- Harder, C.B.; Læssøe, T.; Frøslev, T.G.; Ekelund, F.; Rosendahl, S.; Kjøller, R. A three-gene phylogeny of the Mycena pura complex reveals 11 phylogenetic species and shows ITS to be unreliable for species identification. Fungal Biol. 2013, 117, 764–775. [Google Scholar] [CrossRef]
- Liu, Z.; Na, Q.; Cheng, X.; Wu, X.; Ge, Y. Mycena yuezhuoi sp. nov. (Mycenaceae, Agaricales), a purple species from the peninsula areas of China. Phytotaxa 2021, 511, 148–162. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, Y.; Zeng, H.; Cheng, X.; Na, Q. Four new species of Mycena sect. Calodontes (Agaricales, Mycenaceae) from northeast China. MycoKeys 2022, 93, 23–56. [Google Scholar] [CrossRef]
- Maas Geesteranus, R.A. Mycenas of the Northern Hemisphere. II. Conspectus of the Mycenas of the Northern Hemisphere; North-Holland: Amsterdam, The Netherlands, 1992; 493p. [Google Scholar]
- Maas Geesteranus, R.A.; Horak, E. Mycena and related genera from Papua New Guinea and New Caledonia. Taxonomic Monographs of Agaricales. Bibl. Mycol. 1995, 159, 143–229. [Google Scholar]
- Maas Geesteranus, R.A.; de Meijer, A.A.R. Mycenae Paranaenses; Koninklijke Nederlandse Akademie van Wetenschappen Verhandelingen, Afd. Natuurkunde, Tweede Reeks: Amsterdam, The Netherlands, 1997; 164p. [Google Scholar]
- Métrod, G. Les Mycenes de Madagascar. Prodr. Flore Mycol. Madag. 1949, 3, 1–466. [Google Scholar]
- Olariaga, I.; Pérez-de-Gregorio, M.A.; Arrillaga, P. Porpoloma aranzadii is a synonym of Mycena dura further notes in Mycena sect. Calodontes. Cryptogam. Mycol. 2015, 36, 253–264. [Google Scholar] [CrossRef]
- Clémençon, H. Les rhizomorphes de Prunulus (Basidiomycètes, Tricholomatales, Mycenaceae). Bull. Soc. Mycol. Fr. 2004, 120, 25–35. [Google Scholar]
- Cortés-Pérez, A.; Ramírez-Guillén, F.; Medel, R.; Rockefeller, A. First record of bioluminescence in fungi from Mexico. Mycotaxon 2017, 132, 611–619. [Google Scholar] [CrossRef]
- Cortés-Pérez, A.; Desjardin, D.E.; Perry, B.A.; Ramírez-Cruz, V.; Ramírez-Guillén, F.; Villalobos-Arámbula, A.R.; Rockefeller, A. New species and records of bioluminescent Mycena from Mexico. Mycologia 2019, 111, 319–338. [Google Scholar] [CrossRef]
- Ramírez-Cruz, V.; Cabarroi-Hernández, M.; Villalobos-Arámbula, A.R.; Castro-Jauregui, O.; Cortés-Pérez, A.; Ramírez-Guillén, F.; Zarco-Velazco, G.; Guzmán-Dávalos, L. Records of lignicolous agaricoid fungi (Agaricales, Basidiomycota) from Mexico. Lilloa 2022, 59, 219–271. [Google Scholar] [CrossRef]
- Guzmán, G. Identificación de Los Hongos Comestibles, Venenosos, Alucinantes Y Destructores de la Madera; Limusa: Mexico city, Mexico, 1977; 452p. [Google Scholar]
- Cuevas, R.; Núñez, N.; Sánchez, E. Flora arbórea de La Estación Científica Las Joyas Y Áreas Adyacentes, Sierra de Manantlán, México, 1st ed.; Grupo Editorial Universidad de Guadalajara & Centro Universitario de la Costa Sur: Guadalajara, Mexico, 2021; 456p. [Google Scholar]
- López-Álvarez, R.L.; Luna-Cavazos, M.; Valdez-Hernández, J.I.; García-Moya, E. Tree structure and diversity of a humid mountain forest in the protected natural area La Martinica, Veracruz, Mexico. Rev. Biol. Trop. 2021, 69, 1189–1203. [Google Scholar] [CrossRef]
- Thiers, B.M. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff; New York Botanical Garden’s Virtual Herbarium: New York, NY, USA; Available online: http://sweetgum.nybg.or/science/ih/ (accessed on 1 July 2023).
- Largent, D.; Johnson, D.; Watling, R. How to Identify Mushrooms to Genus III: Microscopic Features; Mad River Press: Eureka, CA, USA, 1977; 166p. [Google Scholar]
- Vellinga, E.C. Glossary. In Flora Agaricina Neerlandica Vol. I; Bas, C., Kuyper, T.W., Noordeloos, M.E., Vellinga, E.C., Eds.; AA Balkema: Rotterdam, The Netherlands, 1988; pp. 54–64. [Google Scholar]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour; Methuen Eyre: London, UK, 1978; 252p. [Google Scholar]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White., T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and TEF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–88. [Google Scholar] [PubMed]
- Chromas vs. 2.6.6 (32 Bit); Technelysium Pty Ltd.: Queensland, Australia, 2018.
- Müller, J.; Müller, K.; Neinhuis, C.; Quand, D. PhyDE—Phylogenetic Data Editor; Version 0.9971; PhyDE, 2010; Available online: http://www.phyde.de/index.html (accessed on 1 January 2023).
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. RaxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree 1.4.1. 2011. Available online: https://tree.bio.ed.ac.uk/software/figtree (accessed on 1 July 2023).








| Taxon | Section | Distribution | Bioluminescence | Reference |
|---|---|---|---|---|
| Mycena fulgoris Cortés-Pérez & Desjardin | Rubromarginatae Singer ex Maas Geest. | Mexico | Basidiome | [28] |
| M. globulispora Maas Geest. & de Meijer | Supinae Konrad & Maubl. | Brazil, Mexico | Basidiome | [28] |
| M. guzmanii Cortés-Pérez, Desjardin & B.A. Perry | Euspeirea (Berk. & Curt.) Sacc. | Mexico | Mycelium and basidiome | [28] |
| M. lumina Cortés-Pérez, Desjardin & A. Rockefeller | Rubromarginatae | Mexico | Mycelium and basidiome | [28] |
| M. luxarboricola Desjardin, B. A. Perry & Stevani | Supinae | Brazil, Mexico | Mycelium and basidiome | [29] |
| M. luxfoliicola Cortés-Pérez, Desjardin & Ram.-Cruz | Nigrescentes Maas Geest. & de Meijer | Mexico | Mycelium and basidiome | [28] |
| M. nebula Cortés-Pérez, Desjardin & A. Rockefeller | Sanguinolentae Maas Geest. | Mexico | Basidiome | [28] |
| M. perlae Cortés-Pérez, Desjardin & A. Rockefeller | Amparoina T. Bau & Q. Na | Mexico | Basidiome | [28] |
| M. stylobates (Pers.) P. Kumm. | Basipedes (Fr.) Quél. | Europe, Mexico, USA | Mycelium and basidiome | [27] |
| Panellus stipticus (Bull.) P. Karst. | Asia, Europe, North America | Mycelium and basidiome | [27] |
| Species | Specimen Voucher | Country | GenBank Accession | References | ||
|---|---|---|---|---|---|---|
| ITS | rpb1 | Tef-1α | ||||
| Mycena aff. pura | TL8052 | Ecuador | FN394623 | KF723687 | KF723641 | [16,18] |
| M. aff. pura | TL9433 | Ecuador | FN394622 | KF723688 | KF723642 | [16,18] |
| M. aff. pura | TL9450 | Ecuador | KJ144653 | KF723689 | KF723643 | [16,18] |
| M. aff. pura | TL9678 | Ecuador | FN394621 | KF723690 | KF723644 | [16,18] |
| M. brunneoviolacea | BAP594, holotype | São Tomé and Príncipe | MH414546 | – | – | [14] |
| M. brunnea | CBH187 | Denmark | FN394564 | KF723678 | KF723632 | [16,18] |
| M. brunnea | CBH386 | Denmark | FN394565 | KF723679 | KF723633 | [16,18] |
| M. cahaya | ACL134, holotype | Malaysia | KF537248 | – | – | [1] |
| M. cf. pura I | CBH039 | Denmark | FN394588 | KF723680 | KF723634 | [16,18] |
| M. cf. pura II | CBH105 | Denmark | FN394581 | KF723671 | KF723625 | [16,18] |
| M. cf. pura II | CBH169 | Denmark | FN394579 | KF723672 | KF723626 | [16,18] |
| M. cf. pura II | CBH366 | Denmark | FN394572 | KF723673 | KF723627 | [16,18] |
| M. cf. pura II | CBH404 | Denmark | FN394566 | KF723674 | KF723628 | [16,18] |
| M. cf. pura III | CBH019 | Denmark | FN394605 | KF723675 | KF723629 | [16,18] |
| M. cf. pura III | CBH022 | Denmark | FN394574 | KF723676 | KF723630 | [16,18] |
| M. cf. pura III | KK | Slovakia | FN394606 | KF723677 | KF723631 | [16,18] |
| M. cf. pura IV | CBH410 | Denmark | FN394595 | KF723667 | KF723621 | [16,18] |
| M. cf. pura IV | JV06979 | Denmark | FN394585 | KF723668 | KF723622 | [16,18] |
| M. cf. pura IV | TL4571 | Denmark | FN394583 | KF723669 | KF723623 | [16,18] |
| M. cf. pura IV | TL12786 | Sweden | FN394591 | KF723670 | KF723624 | [16,18] |
| M. cf. pura VI | BAP132 | USA | FN394561 | KF723660 | KF723614 | [16,18] |
| M. cf. pura VII | IS10/11/200 | USA | FN394611 | – | – | [16,18] |
| M. cf. pura VIII | CBH216 | Denmark | FN394598 | KF723662 | KF723616 | [16,18] |
| M. cf. pura VIII | CBH402 | Denmark | FN394599 | KF723663 | KF723617 | [16,18] |
| M. cf. pura IX | CBH166 | Denmark | FN394607 | KF723701 | KF723655 | [16,18] |
| M. cf. pura IX | CBH358 | Denmark | FN394608 | KF723702 | KF723656 | [16,18] |
| M. cf. pura IX | CBH367 | Denmark | KF913022 | KF723703 | KF723657 | [16,18] |
| M. cf. pura IX | CBH371 | Denmark | KF913023 | KF723704 | KF723658 | [16,18] |
| M. cf. pura X | BAP165A | USA | FN394563 | KF723698 | KF723652 | [16,18] |
| M. diosma | CBH400 | Denmark | FN394617 | KF723699 | KF723653 | [16,18] |
| M. diosma | LK1191/2000 | Germany | FN394619 * | KF723700 | KF723654 | [16,18] |
| M. dura | 10315, holotype | Austria | FN394560 | KF723694 | KF723648 | [16,18] |
| M. lammiensis | TUR165927 | Finland | FN394552 | KF723697 | KF723651 | [16,18] |
| M. luceata | ACP2116, holotype | Mexico | OR233614 | OR233746 | OR233755 | This study |
| M. luceata | ACP2126 | Mexico | OR233613 | OR233745 | OR233754 | This study |
| M. luciferina | ACP2114, holotype | Mexico | OR233612 | OR233744 | – | This study |
| M. lucisnieblae | ACP2140, holotype | Mexico | OR233610 | OR233742 | OR233752 | This study |
| M. lucisnieblae | ACP2139 | Mexico | OR233611 | OR233743 | OR233753 | This study |
| M. lucisnieblae | ACP2149 | Mexico | OR233609 | OR233741 | OR233751 | This study |
| M. lucisnieblae | ACP2166 | Mexico | OR233607 | OR233740 | – | This study |
| M. lucisnieblae | ACP2352-B | Mexico | OR233608 | – | OR233756 | This study |
| M. luteovariegata | CBH226, holotype | Denmark | FN394604 | KF723664 | KF723618 | [16,18] |
| M. luteovariegata | TL5614 | Denmark | FN394602 | KF723666 | KF723620 | [16,18] |
| M. luteovariegata | DB2005/152 | Denmark | FN394603 | – | – | [16,18] |
| M. luxmanantlanensis | ACP2160, holotype | Mexico | OR233603 | OR233737 | OR233747 | This study |
| M. luxmanantlanensis | ACP2159 | Mexico | OR233604 | OR233738 | OR233748 | This study |
| M. pearsoniana | CBH068 | Germany | FN394614 | KF723691 | KF723645 | [16,18] |
| M. pearsoniana | LK880/2002 | Germany | FN394613 | KF723693 | KF723647 | [16,18] |
| M. pelianthina | CBH015 | Denmark | FN394549 | KF723695 | KF723649 | [16,18] |
| M. pelianthina | CBH016 | Denmark | FN394547 | KF723696 | KF723650 | [16,18] |
| M. polycystidiata | FFAAS0417, holotype | China | ON427731 | ON468456 | ON468469 | [20] |
| M. polycystidiata | FFAAS0421 | China | ON427733 | ON468458 | ON468471 | [20] |
| M. rosea | CBH097 | Denmark | FN394556 | KF723681 | KF723635 | [16,18] |
| M. rosea | CBH409 | Germany | FN394551 | KF723683 | KF723637 | [16,18] |
| M. rufobrunnea | FFAAS0414 | China | ON427728 | ON468453 | ON468466 | [20] |
| M. rufobrunnea | FFAAS0416, holotype | China | ON427730 | ON468455 | ON468468 | [20] |
| M. rubromarginata | JV362 | Denmark | FN394624 | KF723705 | KF723659 | [16,18] |
| M. seminau | ACL136, holotype | Malaysia | KF537250 | – | – | [1] |
| M. seminau | ACL308 | Malaysia | KF537252 | – | – | [1] |
| M. shengshanensis, holotype | FFAAS0424 | China | ON427739 | ON468464 | ON468477 | [20] |
| M. shengshanensis | FFAAS0425 | China | ON427740 | ON468465 | ON468478 | [20] |
| M. sinar | ACL092 | Malaysia | KF537247 | – | – | [1] |
| M. sinar | ACL135, holotype | Malaysia | KF537249 | – | – | [1] |
| M. sinar var. tangkaisinar | ACL307, holotype | Malaysia | KF537251 | – | – | [1] |
| M. sophiae | ACP2157, holotype | Mexico | OR233606 | OR233739 | OR233749 | This study |
| M. sophiae | ACP2161 | Mexico | OR233605 | – | OR233757 | This study |
| M. subulata | FFAAS0419 | China | ON427735 | ON468460 | ON468473 | [20] |
| M. subulata | FFAAS0423, holotype | China | ON427737 | ON468462 | ON468475 | [20] |
| M. yuezhuoi | FFAAS0344 | China | MW581490 | MW868166 | MW882249 | [19] |
| M. yuezhuoi | FFAAS0347 | China | MW581493 | MW868167 | MW882252 | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Pérez, A.; Guzmán-Dávalos, L.; Ramírez-Cruz, V.; Villalobos-Arámbula, A.R.; Ruiz-Sanchez, E.; Ramírez-Guillén, F. New Species of Bioluminescent Mycena Sect. Calodontes (Agaricales, Mycenaceae) from Mexico. J. Fungi 2023, 9, 902. https://doi.org/10.3390/jof9090902
Cortés-Pérez A, Guzmán-Dávalos L, Ramírez-Cruz V, Villalobos-Arámbula AR, Ruiz-Sanchez E, Ramírez-Guillén F. New Species of Bioluminescent Mycena Sect. Calodontes (Agaricales, Mycenaceae) from Mexico. Journal of Fungi. 2023; 9(9):902. https://doi.org/10.3390/jof9090902
Chicago/Turabian StyleCortés-Pérez, Alonso, Laura Guzmán-Dávalos, Virginia Ramírez-Cruz, Alma Rosa Villalobos-Arámbula, Eduardo Ruiz-Sanchez, and Florencia Ramírez-Guillén. 2023. "New Species of Bioluminescent Mycena Sect. Calodontes (Agaricales, Mycenaceae) from Mexico" Journal of Fungi 9, no. 9: 902. https://doi.org/10.3390/jof9090902