当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Different Behavior of the Histidine Residue toward Cadmium and Zinc in a Cadmium-Specific Metallothionein from an Aquatic Fungus
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.inorgchem.0c02171 Monica Perinelli 1 , Matteo Tegoni 2 , Eva Freisinger 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.inorgchem.0c02171 Monica Perinelli 1 , Matteo Tegoni 2 , Eva Freisinger 1
Affiliation
|
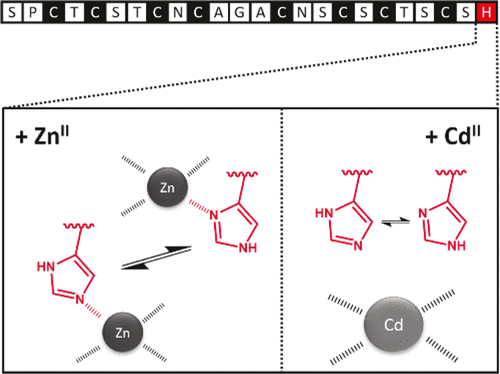
Metallothioneins (MTs) are a large superfamily of ubiquitous cysteine-rich metalloproteins with main functions in metal ion homeostasis and detoxification. Neclu_MT1 is a metallothionein from the aquatic fungus Heliscus lugdunensis and so far the only known MT that is solely induced by CdII but not by ZnII or copper ions. In addition to eight cysteine residues, Neclu_MT1 also contains a less common single C-terminal histidine residue. To better understand the role of this histidine residue in metal ion binding, for the first time, potentiometric pH titrations are applied, revealing insights into the protonation and metal ion binding processes. Additional studies with absorption and NMR spectroscopy complement the finding that while the histidine residue is not crucial for the overall metal binding capacity, it does serve as a ligand in the ZnII but not in the CdII form of the protein.
中文翻译:

在水生真菌特定于镉的金属硫蛋白中,组氨酸残基对镉和锌的不同行为
金属硫蛋白(MTs)是一个普遍存在的富含半胱氨酸的金属蛋白的大家族,在金属离子的稳态和排毒中起主要作用。Neclu_MT1是一种来自水生真菌Heliscus lugdunensis的金属硫蛋白,也是迄今为止唯一已知的仅由Cd II诱导而不由Zn II诱导的MT。或铜离子。除八个半胱氨酸残基外,Neclu_MT1还包含较少见的单个C端组氨酸残基。为了更好地了解该组氨酸残基在金属离子结合中的作用,首次采用电位pH滴定法,揭示了对质子化和金属离子结合过程的见解。利用吸收光谱和NMR光谱进行的其他研究补充了以下发现:组氨酸残基对整体金属结合能力并不重要,但它确实可以作为Zn II的配体而不是以Cd II的形式存在。
更新日期:2020-12-07
中文翻译:

在水生真菌特定于镉的金属硫蛋白中,组氨酸残基对镉和锌的不同行为
金属硫蛋白(MTs)是一个普遍存在的富含半胱氨酸的金属蛋白的大家族,在金属离子的稳态和排毒中起主要作用。Neclu_MT1是一种来自水生真菌Heliscus lugdunensis的金属硫蛋白,也是迄今为止唯一已知的仅由Cd II诱导而不由Zn II诱导的MT。或铜离子。除八个半胱氨酸残基外,Neclu_MT1还包含较少见的单个C端组氨酸残基。为了更好地了解该组氨酸残基在金属离子结合中的作用,首次采用电位pH滴定法,揭示了对质子化和金属离子结合过程的见解。利用吸收光谱和NMR光谱进行的其他研究补充了以下发现:组氨酸残基对整体金属结合能力并不重要,但它确实可以作为Zn II的配体而不是以Cd II的形式存在。






























 京公网安备 11010802027423号
京公网安备 11010802027423号