WO1999060090A1 - Premix composition for clarifying beer - Google Patents
Premix composition for clarifying beer Download PDFInfo
- Publication number
- WO1999060090A1 WO1999060090A1 PCT/US1999/009953 US9909953W WO9960090A1 WO 1999060090 A1 WO1999060090 A1 WO 1999060090A1 US 9909953 W US9909953 W US 9909953W WO 9960090 A1 WO9960090 A1 WO 9960090A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- premix
- premix composition
- xerogel
- particle size
- beer
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12H—PASTEURISATION, STERILISATION, PRESERVATION, PURIFICATION, CLARIFICATION OR AGEING OF ALCOHOLIC BEVERAGES; METHODS FOR ALTERING THE ALCOHOL CONTENT OF FERMENTED SOLUTIONS OR ALCOHOLIC BEVERAGES
- C12H1/00—Pasteurisation, sterilisation, preservation, purification, clarification, or ageing of alcoholic beverages
- C12H1/02—Pasteurisation, sterilisation, preservation, purification, clarification, or ageing of alcoholic beverages combined with removal of precipitate or added materials, e.g. adsorption material
- C12H1/04—Pasteurisation, sterilisation, preservation, purification, clarification, or ageing of alcoholic beverages combined with removal of precipitate or added materials, e.g. adsorption material with the aid of ion-exchange material or inert clarification material, e.g. adsorption material
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12H—PASTEURISATION, STERILISATION, PRESERVATION, PURIFICATION, CLARIFICATION OR AGEING OF ALCOHOLIC BEVERAGES; METHODS FOR ALTERING THE ALCOHOL CONTENT OF FERMENTED SOLUTIONS OR ALCOHOLIC BEVERAGES
- C12H1/00—Pasteurisation, sterilisation, preservation, purification, clarification, or ageing of alcoholic beverages
- C12H1/02—Pasteurisation, sterilisation, preservation, purification, clarification, or ageing of alcoholic beverages combined with removal of precipitate or added materials, e.g. adsorption material
- C12H1/04—Pasteurisation, sterilisation, preservation, purification, clarification, or ageing of alcoholic beverages combined with removal of precipitate or added materials, e.g. adsorption material with the aid of ion-exchange material or inert clarification material, e.g. adsorption material
- C12H1/0408—Pasteurisation, sterilisation, preservation, purification, clarification, or ageing of alcoholic beverages combined with removal of precipitate or added materials, e.g. adsorption material with the aid of ion-exchange material or inert clarification material, e.g. adsorption material with the aid of inorganic added material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/10—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising silica or silicate
- B01J20/103—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising silica or silicate comprising silica
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/22—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising organic material
- B01J20/26—Synthetic macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12C—BEER; PREPARATION OF BEER BY FERMENTATION; PREPARATION OF MALT FOR MAKING BEER; PREPARATION OF HOPS FOR MAKING BEER
- C12C5/00—Other raw materials for the preparation of beer
Definitions
- This invention relates to clarification of beverages such as beer and wine, and, more particularly, to a premix composition and process for effecting such clarification in an efficient and advantageous single-step process.
- Non-biological haze in unstabilized beer arises from complexation of haze-sensitive proteins and haze-producing polyphenols and tannoids. Accordingly, silica gels such as hydrogel or xerogel have been used for effecting clarification of beer by adsorbing haze-sensitive proteins.
- silica hydrogel contains greater than 30% water and is therefore prone to microbial growth on storage.
- Silica xerogel contains only 5% water but becomes compacted upon hydration.
- Crosslinked polyvinylpyrrolidone (PVPP) also has been effective for treating unstabilized beer by specific adsorption of condensed and polymeric polyphenols and tannoids present in beer.
- Patents 2,316,241 ; 3,117,004; 3,163,538; 3,413,120; 3,512,987; 3,554,759; 3,617,301 ; 3,818,111 ; 3,903,316; 4,166,141 ; 4,820,420; 4,910,182; and by the following foreign patents and technical publications:
- Another object herein is to provide a stable premix composition for clarification of beer or wine which has a long shelf life and is not prone to microbiological contamination.
- Still another object of the invention is to provide a stable premix composition of a siliceous material and a crosslinked polyvinyl lactam which is effective for colloidal stabilization of beer.
- Yet another object herein is to provide a process for colloidal stabilization of beer in a single dosing and a single filtration operation.
- a specific object herein is to provide a stable premix composition which is selective to removal of high molecular weight proteins while leaving the desirable low molecular weight proteins remaining in the clarified beer.
- a feature of the present invention is the provision of a stable premix of predetermined composition which is a siliceous xerogel material having less than about 10% by weight of water therein, and a particle size as defined by its mean volume average diamter, Mv, of less than 50 ⁇ , both in the dry state and as a 10% aqueous slurry, and a crosslinked polyvinyl lactam, preferably crosslinked polyvinylpyrrolidone (PVPP), in a weight ratio of about 40 to 90% of the xerogel to about 10 to 60% of PVPP, for effective clarification of beer.
- a stable premix of predetermined composition which is a siliceous xerogel material having less than about 10% by weight of water therein, and a particle size as defined by its mean volume average diamter, Mv, of less than 50 ⁇ , both in the dry state and as a 10% aqueous slurry, and a crosslinked polyvinyl lactam, preferably crosslinked polyvinyl
- a premix composition for clarifying beer in an effective manner which comprises, by weight, (a) 40 to 90% silica xerogel having less than 10% water therein, preferably 5% or less, and (b) 10 to 60% by weight of crosslinked polyvinylpyrrolidone (PVPP).
- PVPP crosslinked polyvinylpyrrolidone
- (a) is 60 to 85% and (b) is 15 to 40%; most preferably, (a) is 70 to 80% and (b) is 20 to 30%.
- component (b) has a particle size as defined by its mean volume average diameter, Mv, in both the dry state and as a 10% aqueous slurry, of less than 50 ⁇ , preferably about 5-30 ⁇
- component (b) has a defined particle size in the dry state of about 20 to 50 ⁇ , and, in a 10% aqueous slurry, of about 30 to 90 ⁇ .
- a premix composition wherein prior to admixture, the ratio between particle sizes of (a) in a 10% aqueous slurry to its dry state is about 0.6 to about 2.0.
- a premix composition wherein prior to admixture, the ratio between the particle sizes of (b) in a 10% aqueous slurry to the dry state is about 1.0 to about 2.0.
- a feature of the invention is the provision of a flocculated aqueous slurry of the defined premix composition, preferably including about 5 to about 20% by wt. of the premix composition and about 80 to about 95% water, for example, which is prepared by admixing silica xerogel and PVPP in defined proportions, and slowly adding water thereto with agitation.
- Another feature of the invention is the provision of a process for clarifying beer which includes treating beer with such an agitated flocculated aqueous slurry of the defined premix, and filtering the thus-treated beer, wherein both proteins and polyphenols are removed in one step from the treated beer in a contact time of about 3 hours or less.
- Such a process requires only a dose of about 10 lbs. of the premix composition for each 100 barrels of beer.
- the process also features a step of conveniently pumping both the clarified beer and the spent premix composition out of the treatment tank into a filter tank after carrying out the clarification step.
- the clarified beer or wine is obtained herein in a process which is conducted at an advantageous filter flow rate, with undetectable residual soluble polyvinylpyrrolidone therein, and no biological growth in the premix, with effective haze stability after time, and easy redispersibility of the used premix.
- FIGURE is a graphical representation of dispersibility of premix compositions of silica xerogel and PVPP as a function of composition.
- Silica gel is produced by reacting sodium silicate with sulfuric acid. The gel then is broken up, washed and sized. This product is known as silica "hydrogel".
- Sodium sulfate is a by-product of the process of formation of silica hydrogel. When sodium sulfate is removed from silica hydrogel and the residue is dried to less than 10% water therein, a silica product known as "xerogel” is obtained.
- a stable premix composition is provided which includes a predetermined composition of xerogel having less than 10% water therein, preferably 5% or less. Suitable xerogels for use herein include SIL- Proof® BG-5 and BG-6 (SCM Chemicals); Britesorb® D-300 (PQ Corp.), and Lucilite XLC (Crossfield Corp.).
- the other component of the premix composition is crosslinked polyvinylpyrrolidone (PVPP), such as Polyclar® PC-10, which is available from International Specialty Products (ISP).
- PVPP crosslinked polyvinylpyrrolidone
- ISP International Specialty Products
- the premix composition for colloidal stabilization of beer is prepared by admixing xerogel and crosslinked polyvinylpyrrolidone (PVPP) solids.
- Suitable premix compositions in accordance with the invention contain about 40 to 90%) by weight of xerogel, preferably 60 to 85%, and most preferably about 70-80%; and about 10 to 60% of PVPP, preferably about 15 to 40%, and most preferably about 20 to 30%.
- the xerogel component provides the larger surface area to receive the PVPP component in a predetermined ratio without causing compactation of the resultant admixture.
- suitable xerogel: PVPP wt. ratios in the premix composition generally will depend upon the particle size of the xerogel used therein.
- Suitable specific premix compositions herein include, for example, 83% xerogel and 17% PVPP (a 15:3 wt. ratio); 70% xerogel and 30% PVPP (a 7:3 wt. ratio); and 63% xerogel with 37%> PVPP (a 1:7 wt. ratio).
- the xerogel component of the premix should have a smaller particle size than the PVPP so that it can be complexed between the PVPP particles.
- the premixed composition can be stored in a stable condition for prolonged periods of time with minimal chance of microbial contamination.
- the premix composition Before use, the premix composition must be hydrated with water with agitation to form an aqueous dispersion or slurry having a premix concentration of about 5-20 wt. %.
- PVPP stabilizes the xerogel by flocculating the xerogel without affecting the requisite adsorbing characteristics of each material.
- This flocculated, aqueous dispersion then is used in a single-step treatment of unstabilized beer. During this treatment, the flocculated premix in the dispersion remains in the slurry state without any significant compaction.
- This stable, flocculated aqueous premix slurry is achieved herein because its PVPP component quickly hydrates upon addition of water thereto to form a swelled system.
- the swelled PVPP system then immediately complexes the xerogel component to prevent premature compaction of the system while the xerogel becomes fully hydrated. Then, in this complexed condition, the xerogel can become fully hydrated by addition of water to the premix over a long period of time without causing compaction of the system.
- the solid premix composition of the invention usually is hydrated with water for about 3 hours to form a thick, flocculated aqueous slurry containing about 5-20 wt. % of the premix.
- This flocculated composition can be kept in a holding tank for long periods without affecting the clarifying properties of either component, and with advantageous microbiological stability.
- the flocculated hydrated premix slurry thus-prepared then is pumped into the beer treatment tank where it can perform its clarifying and chill haze stability functions. After treatment, the clarified beer is pumped into a filter tank then the stabilized beer is passed through a cake of diatomaceous earth to remove any traces of the premix remaining in the beer. Alternate filtration systems like ceramic candles, membrane filtration or centrifugation can be used in the place of diatomaceous earth filtration.
- the advantageous clarification results are achieved herein in a single dosing step with about a 2-30 minute contact time with the two component premix composition of the invention and a single filtration step, operating with an efficient Filter Index, i.e. less pressure build-up across the filter, less diatomaceous earth in the filtration step and a greater beer volume throughput through the filter.
- the stabilized and filtered beer obtained herein had a shelf- life of greater than 3 months, which was over 3 times that of beer treated with either single component of the premix, and equal to sequential single treatments with each component.
- the FIGURE shows the effective dispersibility of xerogel and PVPP premix systems as a function of its composition.
- the degree of dispersibility in an aqueous premix at a 10-20 wt. % concentration is inversely related to the number of inversions required to redisperse a slurry of given composition which has stood for 24 hours.
- Suitable premix compositions require less than 1000 inversions, preferably less than 500 inversions, and, most preferably less than 100 inversions.
- these properties are achieved in premix compositions which contain about 10-60% by weight PVPP (Polyclar 10), preferably 15-40%, and, most preferably, about 20-30%, the rest being the defined silica xerogel component.
- Tannoids are defined as those fractions of the polyphenolic compounds that can be precipitated by the addition of PVP K90 to the beer sample. They include the low and medium molecular weight polyphenols.
- the haze in beer is fundamentally a complex between the condensed polyphenols, referred to as TANNOIDS (T), and the SENSITIVE PROTEINS (P), in an equilibrium governed by the law of mass action as shown in equation (1) and equation (2):
- [P] is the concentration of polypeptides and proteins (Sensitive Proteins defined as substances giving haze when tannin is added) and [T] is the concentration of tannoids that form precipitate with PVP K 90 (molecular weight 350,000).
- Tannoids For the analysis of Tannoids, a solution of PVP K90 was injected into a beer sample. The Tannoids in the beer form a precipitate with PVP K90 through hydrogen bonding. The addition of PVP K90 is plotted against the formation of haze and the maxima of the peak gives the Tannoid Content expressed as mg PVP/L beer.
- the sensitive protein test via the Tannometer provides insight to the levels of haze forming proteins present in beer.
- a solution of tannic acid was dosed into a beer sample. Proteins in the beer complex with tannin to form an insoluble PT complex giving rise to haze. The result is expressed in EBC units of haze corresponding to the addition of 10mg of tannin per liter of beer.
- a lower value of sensitive proteins in the treated beer indicates a reduction in haze.
- the flavanoid content in beer samples was analyzed by Analytica EBC, method 9.9.2.
- Total polyphenols in beer is analyzed using Methods of Analysis of ASBC, method BEER - 35. Both methods give an absorbance value measured by a spectrometer and the results are expressed in ppm.
- HPLC with dual-electrode offers a precise qualitative and quantitative method for the determination of haze producing flavanols in beer.
- the flavanoid/polyphenols in beer are of two-fold interest, owing to their proven involvement in haze formation and their potential impact on flavor. Malt and hops provide beer with its share of the polyphenols.
- the total haze is read directly from the bottle, using an Lg automatic haze meter.
- the haze meter is calibrated with certified haze standards obtained from Advanced Polymer Systems. All readings are taken with distilled water in the measuring chamber to prevent the formation of condensation on the outside surface of cold samples.
- Haze readings are taken on fresh beer samples at 22°C and at 0°C. Aging tests are performed by incubating samples in a dry oven at 37°C for one week and then transferring to storage at 0°C for one day before taking total haze readings on the cold samples. Samples are put through this cycle for several weeks or until an excessive value for haze is obtained. The end of useful shelf life is generally taken to be 2.0 EBC haze units and one week storage at 37°C is taken as being equal to one-month storage at ambient temperature.
- Runs 1-8 are Lab Runs. Runs 1 , 2, 3, 4 are Comparative Runs. Runs 5 and 6 are Invention Runs. Runs 7 and 8 are Control Runs.
- Unstabilized beer was used for Examples 1 through 7. This beer sample was not treated with any form of stabilizer and was centrifuged to decrease yeast cell count to approximately 1 million cells per ml by the brewery.
- a 1500-ml glass jar equipped with a lid was added 1000-ml of unstabilized beer, 0.571g (equivalent to a dosing rate of 15-lbs/100bbl) of Xerogel (Britesorb D-300, PQ Corporation) and a magnetic stir bar. This mixture was placed on a magnetic stir plate within a refrigerator, set at 0°C. After 3 hours of stirring, 1.90g of diatomaceous earth (DE) was added (equivalent to 50-lbs/100bbl) and mixed into the solution by swirling the jar.
- DE diatomaceous earth
- Run 1 was repeated except that 0.267g of Polyclar ® 10 (equivalent to 7-lbs/100bbl) was added in place of 0.114g of Polyclar ® 10 after the first filtration process. Results can be found in Tables 1 , 2 and 3.
- Example 2 In an experiment similar to that performed in Example 1 , a 1000-ml sample of unstabilized beer was dosed with 0.571g of Xerogel (Britesorb D-300, equivalent to 15-lbs/100bbl) and mechanically stirred for 2-3/4 hours. Then, 0.114g of Polyclar ® 10 (equivalent to 3-lbs/1 OObbl) was added to the mixture and stirred for an additional 15 minutes. DE was dosed into the sample and the mixture was filtered as described in Example 1. Results can be found in Tables 1 , 2 and 3.
- Example 3 was repeated except that 0.267g Polyclar ® 10 (equivalent to 7-lbs/100bbl) was used in the place of 0.114g of Polyclar ® 10. Results can be found in Tables 1 , 2 and 3.
- Example 2 In an experiment similar to that performed in Example 1 , Xerogel (Britesorb D-300) and Polyclar ® 10 were premixed in the ratio of 15:3 by weight. A 1000-ml sample of unstabilized beer was dosed with 0.685g of the 15:3 ratio premix (equivalent to 18-lbs/100bbl). The sample was placed on a magnetic stir plate within a refrigerator, set at 0°C. After 3 hours of stirring, 1.90g of diatomaceous earth (DE, equivalent to 50-lbs/100bbl) was added and mixed into the solution by swirling the jar. This mixture was then vacuum filtered through a 2.5- ⁇ m glass fiber filter using a B ⁇ chner funnel and vacuum flask. The filtered beer was then analyzed as described in Example 1. Results can be found in Tables 1 , 2 and 3.
- DE diatomaceous earth
- Example 5 was repeated except that Xerogel (Britesorb D-300) and Polyclar ® 10 were premixed in the ratio of 15:7 by weight. A 1000-ml sample of beer was dosed with 0.838g of the 15:7 ratio premix (equivalent to 22- lbs/1 OObbl) and processed as described in Example 5. Results can be found in Tables 1 , 2 and 3.
- a control experiment was performed by dosing 1000-ml of unstabilized beer with 0.157g of Xerogel (Britesorb D-300, equivalent to 15-lbs/1 OObbl). The mixture was mechanically stirred for 3 hours in a refrigerator, set at 0°C. 1.90g of diatomaceous earth (DE) was added to the mixture (equivalent to 50- lbs/1 OObbl) and mixed into the solution by swirling the jar. This mixture was then vacuum filtered through a 2.5- ⁇ m glass fiber filter using a B ⁇ chner funnel and vacuum flask. The filtered beer was then analyzed as described in Example 1. Results can be found in Tables 1 , 2 and 3.
- DE diatomaceous earth
- a brewery trial for a German Pilsner was carried out by stabilizing 500hl of beer with silica hydrogel alone (a prior art treatment), silica hydrogel plus Polyclar® 10 and the admixture of this invention. All stabilizers were added to the beer prior to filtration with DE (kieselguhr), with an estimated contact time with beer of 10 minutes. Forced haze development was measured by the number of 60°C/0°C cycles (24 hours at each temperature) required to reach 2.0 EBC haze. 8 cycles by the above method is taken as equivalent to 10 months of predicted shelf life.
- Xerogel (Millennium BG5) and Polyclar ® 10 were premixed in the ratio of 7:3 by weight.
- a 1000-ml sample of a new unstabilized beer was dosed with 0.381 g of the 7:3 ratio premix (equivalent to 10-lbs/1 OObbl).
- the sample was mechanically stirred using a magnetic stir plate within a refrigerator, set at 0°C.
- 1.90g of diatomaceous earth (DE, equivalent to 50-lbs/1 OObbl) was added and mixed into the solution by swirling the jar. This mixture was then vacuum filtered through a 2.5- ⁇ m glass fiber filter using a B ⁇ chner funnel and vacuum flask.
- the filtered beer was then analyzed as described in Example 1. Results can be found in Tables 7 and 8 below.
- Example 10 was repeated except that the 7:3 premix was dosed at 0.571 g (equivalent to 15-lbs/1 OObbl). Results can be found in Tables 7 and 8.
- Example 12 was repeated except that 0.762g of Xerogel (Millennium BG5, equivalent to 20-lbs/1 OObbl) was used in place of Polyclar ® 10. Results can be found in Tables 7 and 8.
- Example 12 was repeated except that 0.571 g of Xerogel (Millennium BG5, equivalent to 15-lbs/1 OObbl) was used in place of Polyclar ® 10. Results can be found in Tables 7 and 8. TABLE 7
- Example 10 was repeated except that Xerogel, Lucilite XLC (Crossfield Corp.) was used in place of Xerogel, Millennium BG5. Results can be found in Tables 9 and 10.
- Example 11 was repeated except that Xerogel, Lucilite XLC (Crossfield Corp.) was used in place of Xerogel, Millennium BG5. Results can be found in Tables 9 and 10. EXAMPLE 17 - Polyclar ® 10 Treatment
- Example 12 was repeated. Results can be found in Tables 9 and 10.
- Example 13 was repeated except that Xerogel, Lucilite XLC (Crossfield Corp.) was used in place of Xerogel, Millennium BG5. Results can be found in Tables 9 and 10.
- Example 14 was repeated except that Xerogel, Lucilite XLC (Crossfield Corp.) was used in place of Xerogel, Millennium BG5. Results can be found in Tables 9 and 10.
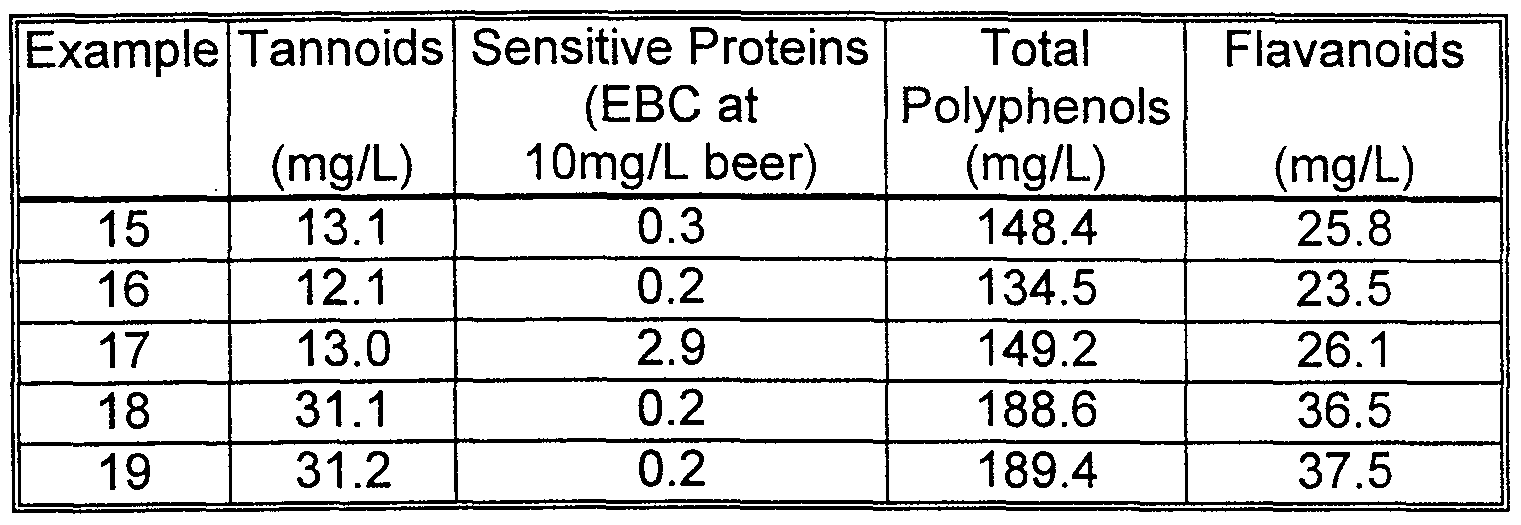
- Example 16 produced far superior stabilization (lower total EBC value) than Comparative Runs 17-19.
- Example 20 was repeated except that 10g of Xerogel (Millennium BG6) was used in place of Polyclar ® 10. Results are tabulated in Table 11.
- Example 20 was repeated except that 10g of Xerogel (Millennium BG5) was used in place of Polyclar ® 10. Results are tabulated in Table 11.
- Example 20 was repeated except that 10g of Xerogel (Crossfield, Lucilite XLC) was used in place of Polyclar ® 10. Results are tabulated in Table 11.
- Example 24 Sedimentation Properties of Polyclar 10/Xerogel (Millennium BG6) Mixture
- Example 20 was repeated except that 10g of Polyclar ® 10 was replaced with a solid premix containing 7g of Xerogel (Millennium BG6) and 3g Polyclar ® 10. Results are tabulated in Table 11.
- Example 20 was repeated except that 10g of Polyclar ® 10 was replaced with a solid premix containing 7g of Xerogel (Millennium BG5) and 3g Polyclar ® 10. Results are tabulated in Table 11.
- EXAMPLE 26 Sedimentation Properties of Poivclar ® 10/Xerogel (Crossfield. Lucilite XLC) Mixture
- Example 20 was repeated except that 10g of Polyclar ® 10 was replaced with a solid premix containing 7g of Xerogel (Crossfield, Lucilite XLC) and 3g Polyclar ® 10. Results are tabulated in Table 11.
- Example 20 was repeated except that 8g of Xerogel (Millennium BG6) and 2g of Polyclar ® 10 were used. Results are tabulated in Table 11.
- Example 20 was repeated except that 8g of Xerogel (Millennium BG5) and 2g of Polyclar ® 10 were used. Results are tabulated in Table 11.
- EXAMPLE 29 Sedimentation Properties of Polvclar ® 10/Xerogel (Crossfield. Lucilite XLC) Mixture
- Example 20 was repeated except that 8g of Xerogel (Crossfield, Lucilite XLC) and 2g of Polyclar ® 10 were used. Results are tabulated in Table 11.
- the following twelve admixtures were prepared by blending xerogel (Britesorb D-300) and increasing quantities of Polyclar 10 containing the following weight % of Polyclar 10. 0%, 8%, 16%o, 25%, 30%, 32%, 42%, 50%, 65%, 75%, 85% and 100%. This was carried out by mixing the components in a V-blender for a period of 60 minutes.
- the filter flow rates for the flow of water over a filter bed prepared from the above admixtures were determined as follows.
- Example 40 The experiment in Example 40 was repeated replacing Britesorb D-300 with BG6.
- the following weight % Polyclar 10 were used in this case, 0%, 17%, 25%, 30%), 32%, 41%, 50%, 65%, 75%, 85%, 90% and 100%.
- the results are tabulated in Table 14.
- Filter Flow Rate maximizes at Polyclar 10 concentration of between 41% and 65 % (premix with BG6.)
- Polyclar 10/Xerogel (BG6) premix was prepared by mixing 70g of BG6 and 30g of Polyclar 10 in a V-blender for a period of 60 minutes. Particle size distribution of the premix was determined and recorded under Column III in Table 15. Later, 10g of this premix was added to a stoppered graduated cylinder. Distilled water was added to bring the volume to the 100ml mark and mixed with the powder to disperse the solids. It was then allowed to stand overnight to fully hydrate the contents in the cylinder. The samples were then re-mixed by vigorous inversions of the cylinder to fully disperse the solids. The samples was then tested for particle size distribution, similar to the DRY sample, by Microtarc - SRA 9200, results are shown under HYDRATED in Table 15.
- Example A1 was repeated, except in this case the Xerogel BG5 was used instead of Xerogel BG6.
- Example 42-A3 was repeated, except in this case the 7:3 premix was made with Xerogel BG5 and Polyclar 10.
- Example 42-A1 was repeated, except in this case the Xerogel Britesorb D-300 was used instead of Xerogel BG6.
- Example 42-A3 was repeated, except in this case the 7:3 premix was made with Xerogel Britesorb D-300 and Polyclar 10.
- Example 42-A1 was repeated, except in this case the Xerogel Lucilite XLC was used instead of Xerogel BG6.
- EXAMPLE 42-D2 was repeated, except in this case the Xerogel Lucilite XLC was used instead of Xerogel BG6.
- Example 42-A3 was repeated, except in this case the 7:3 premix was made with Xerogel Lucilite XLC and Polyclar 10.
- Example 42-A1 was repeated, except in this case the Xerogel Stabifix was used instead of Xerogel BG6.
- Example 42-A3 was repeated, except in this case the 7:3 premix was made with Xerogel Stabifix and Polyclar 10.
- Example 42-A1 was repeated, except in this case the Hydrogel Chillgarde was used instead of Xerogel BG6.
- Example 42-A3 was repeated, except in this case the 7:3 premix was made with Hydrogel Chillgarde and Polyclar 10.
- Example 42-A1 was repeated, except in this case the Hydrogel Britesorb A-100 was used instead of Xerogel BG6.
- Example 42-A3 was repeated, except in this case the 7:3 premix was made with Hydrogel Britesorb A-100 and Polyclar 10. TABLE 15
- the mean volume diameter of the hydrated premix of Xerogel and Polyclar 10 shows a marked increase compared to the individual components. This increase in the particle size under wet conditions is indicative of the flocculent effect of Polyclar 10.
- Polyclar 10/Xerogel (Britesorb D-300) premix was prepared by mixing 150g of Xerogel (Britesorb D-300) and 30g of Polyclar 10 in a V blender for a period of 60 minutes. Similarly, a premix of 150 g of Xerogel (Chillgarde) and 30g of Polyclar 10 was prepared by mixing in a V blender for a period of 60 minutes. These two premixes together with single components of Polyclar 10, Chillgarde and Britesorb D-300 were also, used in the experiment. All the samples were assessed for microbiological stability using the test "Adequacy of Preservation (Challenge) Test" from Sutton Laboratories, Method MLM 100-9. The challenge test protocol is designed to assess effective antimicrobial activity over storage time, thus simulating shelf life of the product.
Abstract
Description
Claims
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP99921745A EP1078036B1 (en) | 1998-05-15 | 1999-05-05 | Premix composition for clarifying beer |
| BRPI9910442-3A BR9910442B1 (en) | 1998-05-15 | 1999-05-05 | Premix composition for effective lightening of beer in a single step process and process for stabilizing beer or wine. |
| AU38872/99A AU757835B2 (en) | 1998-05-15 | 1999-05-05 | Premix composition for clarifying beer |
| DE69928921T DE69928921T2 (en) | 1998-05-15 | 1999-05-05 | PREPARATION COMPOSITION FOR BEER DECLARATION |
| JP2000549698A JP4298920B2 (en) | 1998-05-15 | 1999-05-05 | Premix composition for beer clarification |
| AT99921745T ATE312905T1 (en) | 1998-05-15 | 1999-05-05 | PREMIX COMPOSITION FOR BEER CLARIFICATION |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US7953998A | 1998-05-15 | 1998-05-15 | |
| US09/079,539 | 1998-05-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1999060090A1 true WO1999060090A1 (en) | 1999-11-25 |
Family
ID=22151189
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1999/009953 WO1999060090A1 (en) | 1998-05-15 | 1999-05-05 | Premix composition for clarifying beer |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US7153534B2 (en) |
| EP (1) | EP1078036B1 (en) |
| JP (1) | JP4298920B2 (en) |
| KR (1) | KR100605202B1 (en) |
| CN (1) | CN1211481C (en) |
| AT (1) | ATE312905T1 (en) |
| AU (1) | AU757835B2 (en) |
| BR (1) | BR9910442B1 (en) |
| DE (1) | DE69928921T2 (en) |
| ES (1) | ES2255267T3 (en) |
| WO (1) | WO1999060090A1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008097154A1 (en) * | 2007-02-09 | 2008-08-14 | Ge Healthcare Bio-Sciences Ab | Liquid clarification |
| WO2012011807A1 (en) * | 2010-07-22 | 2012-01-26 | Heineken Supply Chain B.V. | A method of stabilising yeast fermented beverages |
| WO2012011808A1 (en) * | 2010-07-22 | 2012-01-26 | Heineken Supply Chain B.V. | A method for the regeneration of pvpp from a membrane filter retentate after clarification and stabilisation of a yeast fermented beverage |
| US9476020B2 (en) | 2010-07-22 | 2016-10-25 | Heineken Supply Chain B.V. | Method and apparatus for the recovery of PVPP after contact with a yeast fermented beverage by sedimentation separation |
| WO2017109194A1 (en) * | 2015-12-23 | 2017-06-29 | Poromembrane Gmbh | Filter membrane comprising two adsorbents |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10051266A1 (en) * | 2000-10-16 | 2002-04-25 | Basf Ag | Filter aid used for filtering fruit and fermented drinks comprising polystyrene and silicate, carbonate, oxide, silica gel, diatomaceous earth and/or polymers |
| GB0101507D0 (en) * | 2001-01-22 | 2001-03-07 | Crosfield Joseph & Sons | Stabilising beverages |
| DE10215147A1 (en) * | 2002-04-05 | 2003-10-16 | Basf Ag | Use of polymerization containing thermoplastic polymers as filter aids and / or stabilizers |
| US7229655B2 (en) * | 2003-12-30 | 2007-06-12 | Pq Corporation | Composition of, and process for using, silica xerogel for beer stabilization |
| CA2567240C (en) | 2004-05-18 | 2011-12-13 | Inbev S.A. | Method of preparing a liquid, containing proteins for subsequent separation, by using one or more protein-complexing agents |
| EP2022555B1 (en) | 2006-04-26 | 2016-03-09 | Toyobo Co., Ltd. | Polymeric porous hollow fiber membrane |
| WO2008008940A2 (en) * | 2006-07-14 | 2008-01-17 | World Minerals, Inc. | Composition for filtering and removing particles and/or constituents from a fluid |
| PT2049645T (en) | 2006-08-07 | 2016-07-14 | Grace Gmbh & Co Kg | Beer clarification aid based on silica xerogel with high filterability |
| PL2155851T3 (en) * | 2007-06-06 | 2014-12-31 | Basf Se | Use of n-vinylimidazole polymers to improve the value-determining properties of biologic fermented solutions |
| US8409647B2 (en) * | 2008-08-12 | 2013-04-02 | E. I. Du Pont De Nemours And Company | Silica microgels for reducing chill haze |
| EP2358768B1 (en) * | 2008-12-03 | 2019-07-03 | ISP Investments LLC | Crosslinked polyvinylpyrrolidone compositions |
| CN110187050B (en) * | 2018-02-23 | 2023-04-11 | 山西燕京啤酒有限公司 | Detection method for judging quality of tetrahydrochysene bitter water suitable for beer enterprises |
| JP7048791B2 (en) * | 2020-09-10 | 2022-04-05 | アサヒビール株式会社 | Method of suppressing the occurrence of spouting of effervescent fermented malt beverage |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4636394A (en) * | 1984-04-16 | 1987-01-13 | J. E. Siebel Sons' Company, Inc. | Method and compositions for chillproofing beverages |
| US4910182A (en) * | 1985-03-19 | 1990-03-20 | Westfalia Separator Ag | Process for the secondary purification and stabilization of liquids containing polyphenols and/or proteins, particularly beverages and more especially beer |
| US5628910A (en) * | 1996-06-28 | 1997-05-13 | W. R. Grace & Co.-Conn. | Polyamide compositions for removal of polyphenols from liquids |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62207712A (en) * | 1986-03-05 | 1987-09-12 | Fuji Debuison Kagaku Kk | Hydrous silica gel for stabilizing beer |
| US5149553A (en) * | 1989-10-12 | 1992-09-22 | Pq Corporation | Beer processing and composition |
-
1999
- 1999-05-05 ES ES99921745T patent/ES2255267T3/en not_active Expired - Lifetime
- 1999-05-05 KR KR1020007012695A patent/KR100605202B1/en not_active IP Right Cessation
- 1999-05-05 WO PCT/US1999/009953 patent/WO1999060090A1/en active IP Right Grant
- 1999-05-05 DE DE69928921T patent/DE69928921T2/en not_active Expired - Lifetime
- 1999-05-05 AT AT99921745T patent/ATE312905T1/en not_active IP Right Cessation
- 1999-05-05 JP JP2000549698A patent/JP4298920B2/en not_active Expired - Lifetime
- 1999-05-05 CN CNB998060798A patent/CN1211481C/en not_active Expired - Lifetime
- 1999-05-05 EP EP99921745A patent/EP1078036B1/en not_active Expired - Lifetime
- 1999-05-05 AU AU38872/99A patent/AU757835B2/en not_active Expired
- 1999-05-05 BR BRPI9910442-3A patent/BR9910442B1/en not_active IP Right Cessation
-
2003
- 2003-08-26 US US10/648,660 patent/US7153534B2/en not_active Expired - Lifetime
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4636394A (en) * | 1984-04-16 | 1987-01-13 | J. E. Siebel Sons' Company, Inc. | Method and compositions for chillproofing beverages |
| US4910182A (en) * | 1985-03-19 | 1990-03-20 | Westfalia Separator Ag | Process for the secondary purification and stabilization of liquids containing polyphenols and/or proteins, particularly beverages and more especially beer |
| US5628910A (en) * | 1996-06-28 | 1997-05-13 | W. R. Grace & Co.-Conn. | Polyamide compositions for removal of polyphenols from liquids |
Non-Patent Citations (1)
| Title |
|---|
| HARDWICK A. H.: "HANDBOOK OF BREWING, PASSAGE.", HANDBOOK OF BREWING, XX, XX, 1 January 1995 (1995-01-01), XX, pages 231/232., XP002921688 * |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008097154A1 (en) * | 2007-02-09 | 2008-08-14 | Ge Healthcare Bio-Sciences Ab | Liquid clarification |
| US8137559B2 (en) | 2007-02-09 | 2012-03-20 | Ge Healthcare Bio-Sciences Ab | Liquid clarification |
| WO2012011807A1 (en) * | 2010-07-22 | 2012-01-26 | Heineken Supply Chain B.V. | A method of stabilising yeast fermented beverages |
| WO2012011808A1 (en) * | 2010-07-22 | 2012-01-26 | Heineken Supply Chain B.V. | A method for the regeneration of pvpp from a membrane filter retentate after clarification and stabilisation of a yeast fermented beverage |
| EA022584B1 (en) * | 2010-07-22 | 2016-01-29 | Хейнекен Сэпплай Чэйн Б.В. | Method of preparing beverage and apparatus |
| US9476020B2 (en) | 2010-07-22 | 2016-10-25 | Heineken Supply Chain B.V. | Method and apparatus for the recovery of PVPP after contact with a yeast fermented beverage by sedimentation separation |
| US9476021B2 (en) | 2010-07-22 | 2016-10-25 | Heineken Supply Chain B.V. | Method for the regeneration of PVPP from a membrane filter retentate after clarification and stabilization of a yeast fermented beverage |
| US9481859B2 (en) | 2010-07-22 | 2016-11-01 | Heineken Supply Chain B.V. | Method of stabilizing yeast fermented beverages |
| EA025808B1 (en) * | 2010-07-22 | 2017-01-30 | Хейнекен Сэпплай Чэйн Б.В. | Method of stabilising yeast fermented beverages |
| WO2017109194A1 (en) * | 2015-12-23 | 2017-06-29 | Poromembrane Gmbh | Filter membrane comprising two adsorbents |
| US10967332B2 (en) | 2015-12-23 | 2021-04-06 | Pall Corporation | Filter device |
Also Published As
| Publication number | Publication date |
|---|---|
| AU757835B2 (en) | 2003-03-06 |
| JP4298920B2 (en) | 2009-07-22 |
| US20040043119A1 (en) | 2004-03-04 |
| ATE312905T1 (en) | 2005-12-15 |
| US7153534B2 (en) | 2006-12-26 |
| BR9910442B1 (en) | 2010-10-19 |
| EP1078036A1 (en) | 2001-02-28 |
| ES2255267T3 (en) | 2006-06-16 |
| KR20010034856A (en) | 2001-04-25 |
| BR9910442A (en) | 2001-01-02 |
| EP1078036A4 (en) | 2004-05-19 |
| KR100605202B1 (en) | 2006-07-28 |
| AU3887299A (en) | 1999-12-06 |
| CN1300317A (en) | 2001-06-20 |
| DE69928921D1 (en) | 2006-01-19 |
| CN1211481C (en) | 2005-07-20 |
| EP1078036B1 (en) | 2005-12-14 |
| DE69928921T2 (en) | 2006-07-27 |
| JP2002515236A (en) | 2002-05-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU757835B2 (en) | Premix composition for clarifying beer | |
| US20090214701A1 (en) | Method of preventing or reducing haze in a beverage using silane-treated silica filter media | |
| US7993688B2 (en) | Method of preparing a liquid, containing proteins for subsequent separation, by using one or more protein-complexing agents | |
| US4508742A (en) | Treating beer to prevent chill haze and metal contamination | |
| JPH04325078A (en) | Method of eliminating heavy metal ion from wine and winelike beverage | |
| US7767621B2 (en) | Processes for reducing beer soluble iron in diatomaceous earth products, diatomaceous earth products and compositions thereof, and methods of use | |
| RU2281325C2 (en) | Method for beverage stabilization | |
| CA2272032C (en) | Premix composition for clarifying beer | |
| NZ201648A (en) | A composition and method for stabilizing beverages against haze formation | |
| JP3460357B2 (en) | Silicon dioxide for stabilizing beer and method for producing the same | |
| JP6851581B2 (en) | Stabilization of brewed sake | |
| MXPA00011016A (en) | Premix composition for clarifying beer | |
| Dadic et al. | The use of Polyclar AT (PVPP) in brewing | |
| SU1409200A1 (en) | Method of producing instant tea | |
| WO2000066705A2 (en) | Process and composition for reducing chill haze in beverages | |
| RU2330879C2 (en) | Application of colloid anionic silicic sol as clarifier | |
| WO1991019780A1 (en) | Protein removal using precipitated silica | |
| GB2028340A (en) | Finings for beer | |
| WO2010040114A1 (en) | Process for extending beverage stability | |
| Lewis et al. | Haze | |
| JPH0479876A (en) | Stabilization treating agent for beer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 99806079.8 Country of ref document: CN |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AL AM AT AU AZ BA BB BG BR BY CA CH CN CU CZ DE DK EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT UA UG US UZ VN YU ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SL SZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WD | Withdrawal of designations after international publication |
Free format text: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 38872/99 Country of ref document: AU |
|
| ENP | Entry into the national phase |
Ref document number: 2000 549698 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/a/2000/011016 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020007012695 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1999921745 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1999921745 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020007012695 Country of ref document: KR |
|
| WWG | Wipo information: grant in national office |
Ref document number: 38872/99 Country of ref document: AU |
|
| WWG | Wipo information: grant in national office |
Ref document number: 1999921745 Country of ref document: EP |
|
| WWG | Wipo information: grant in national office |
Ref document number: 1020007012695 Country of ref document: KR |